STRATTERA 4 mg/ml ORAL SOLUTION

How to use STRATTERA 4 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Strattera 4mg/ml oral solution
atomoxetine
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Strattera and what is it used for
- What you need to know before you take Strattera
- How to take Strattera
- Possible side effects
- Storing Strattera
- Contents of the pack and other information
1. What is Strattera and what is it used for
What is it used forStrattera contains atomoxetine and is used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in:
- children over 6 years of age
- adolescents
- adults
It is used as part of a comprehensive treatment programme, which typically includes psychological, educational, and social measures.
It is not intended for use in children under 6 years of age as the safety and efficacy in this age group have not been established.
In adults, Strattera is used to treat ADHD when the symptoms are very troublesome and affect your work or social life and when you have had symptoms of the disorder as a child.
How it worksStrattera increases the levels of noradrenaline in the brain. This is a chemical that is produced naturally and increases attention and decreases impulsiveness and hyperactivity in patients with ADHD. This medicine has been prescribed to help control the symptoms of ADHD. This medicine is not a stimulant and it is not addictive. It may take a few weeks before you start to feel the benefits of Strattera.
About ADHDChildren and adolescents with ADHD find it:
- difficult to sit still
- difficult to concentrate
This is not because they are not trying, but because they have difficulty controlling their behaviour. In children and adolescents, ADHD is characterised by a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or relationships. ADHD can make it difficult to pay attention, control behaviour, and sit still. Adults with ADHD find it difficult to do all these things, but this may mean that they have problems with:
- work
- relationships
- low self-esteem
- education
2. What you need to know before you take Strattera
Do not take Strattera if you:
- are allergic to atomoxetine or any of the other ingredients of this medicine (listed in section 6)
- have taken a medicine called a monoamine oxidase inhibitor (MAOI), for example phenelzine, within the last two weeks. MAOIs are sometimes used for depression and other mental health conditions; taking Strattera with an MAOI could cause serious side effects or be life-threatening. You should also wait at least 14 days after stopping MAOI treatment before starting treatment with Strattera.
- have an eye problem called narrow-angle glaucoma (increased pressure in the eye)
- have a serious heart problem that can affect the blood flow, which may get worse with Strattera
- have serious blood vessel problems that can affect blood flow to the brain or heart or that can cause an aneurysm (a bulge in a blood vessel that can burst), or have had such problems in the past
- have a tumour of the adrenal gland (phaeochromocytoma)
Do not take Strattera if any of the above apply to you. If you are not sure, consult your doctor or pharmacist before taking Strattera, as Strattera may make these conditions worse.
Warnings and precautions
Both adults and children should be aware of the following warnings and precautions. Consult your doctor or pharmacist before taking Strattera if you have:
- thoughts of suicide or have tried to harm yourself
- heart problems (including heart defects) or an increased heart rate. Strattera can increase your heart rate (pulse). There have been reports of sudden death in patients with heart defects.
- high blood pressure. Strattera can increase your blood pressure.
- low blood pressure. Strattera can cause dizziness or fainting in people with low blood pressure.
- problems with sudden changes in blood pressure or heart rate
- cardiovascular disease or a history of having had a stroke
- liver problems. You may need a lower dose.
- psychotic symptoms including hallucinations (hearing voices or seeing things that are not there), believing things that are not true, or being suspicious
- mania (feeling elated or over-excited, which can lead to abnormal behaviour) and agitation
- aggressive feelings
- hostility
- a history of epilepsy or have had seizures for any other reason. Strattera may increase the risk of seizures.
- mood changes or feeling unhappy
- repetitive behaviours or sounds or words
Talk to your doctor or pharmacist if you have any of the symptoms mentioned above before starting treatment, as Strattera may make these problems worse. Your doctor will want to keep an eye on how the medicine affects you.
Treatment with Strattera may cause you to feel agitated, hostile, or violent, or cause these symptoms to get worse if they are already present. It may also cause changes in your behaviour or mood (including physical aggression, threatening behaviour, and thoughts of harming others). If you or your family/carer notice any of these reactions, tell your doctor or pharmacist immediately.
Serotonin Syndrome
Serotonin syndrome is a potentially life-threatening condition that may occur when taking Strattera in combination with other medicines (see section 2, "Other medicines and Strattera"). The signs and symptoms of serotonin syndrome can include a combination of the following: confusion, restlessness, lack of coordination, and rigidity, hallucinations, coma, rapid heartbeat, increased body temperature, changes in blood pressure, sweating, flushing, tremors, overactive reflexes, nausea, vomiting, and diarrhoea. Contact a doctor or go to the emergency department of the nearest hospital immediately if you think you are experiencing serotonin syndrome.
Tests your doctor will do before you start taking Strattera
These tests are to decide if Strattera is the right medicine for you.
Your doctor will:
- measure your blood pressure and heart rate (pulse) before and during the time you are taking Strattera
- measure your height and weight if you are a child or adolescent during the time you are taking Strattera
Your doctor will ask you about:
- other medicines you are taking
- any family history of sudden death
- any other medical problems (such as heart problems) that you or your family may have
It is important that you provide all the information you can. This will help your doctor decide if Strattera is the right medicine for you. Your doctor may decide to do other medical tests before starting treatment with this medicine.
Other medicines and Strattera
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This includes medicines that you buy without a prescription. Your doctor will decide if you can take Strattera with other medicines, and may need to adjust the dose or increase it more slowly.
Do not take Strattera with MAOIs (monoamine oxidase inhibitors) taken for depression. See section 2, "Do not take Strattera".
If you are taking other medicines, Strattera may affect how they work, or they may affect how Strattera works. If you are taking any of the following medicines, check with your doctor or pharmacist before taking Strattera:
- medicines that increase blood pressure or are used to control blood pressure
- medicines such as antidepressants, for example imipramine, venlafaxine, mirtazapine, fluoxetine, and paroxetine
- certain cough and cold remedies that contain medicines that can affect blood pressure. When buying these products, it is important to check with your pharmacist
- certain medicines used to treat mental health conditions
- medicines that are known to increase the risk of seizures
- certain medicines that make Strattera stay in the body longer than it normally would (such as quinidine and terbinafine)
- salbutamol (a medicine for asthma) when taken by mouth or injected, may make you feel like your heart is racing, but this will not make your asthma worse
The following medicines may increase the risk of an abnormal heart rhythm when taken with Strattera:
- medicines used to control the heart rhythm
- medicines that alter the levels of salts in the blood
- medicines for the prevention and treatment of malaria
- antibiotics (such as erythromycin and moxifloxacin)
If you are not sure if the medicines you are taking are listed above, consult your doctor or pharmacist before taking Strattera.
Strattera may affect or be affected by other medicines. These include:
- Certain antidepressants, opioids such as tramadol, and medicines used to treat migraine called triptans. These medicines may interact with Strattera and cause serotonin syndrome, a potentially life-threatening condition (see section 2, Warnings and precautions, Serotonin Syndrome)
Pregnancy and breast-feeding
It is not known if this medicine can affect the unborn baby or pass into breast milk.
- This medicine should not be taken during pregnancy unless your doctor advises you to do so.
- You should avoid taking this medicine if you are breast-feeding or stop breast-feeding
If you:
- are pregnant or breast-feeding
- think you may be pregnant or are planning to have a baby
- are planning to breast-feed
consult your doctor or pharmacist before taking this medicine.
Driving and using machines
Strattera may cause drowsiness, dizziness, or sleepiness. Be careful when driving or using machinery until you know how Strattera affects you. If you feel drowsy, dizzy, or sleepy, do not drive or use machinery.
Important information about the oral solution
This oral solution may irritate your eyes. If the oral solution comes into contact with your eyes, rinse them immediately with plenty of water and consult your doctor. If your hands or any other part of your body come into contact with the oral solution, wash them with water as soon as possible.
Strattera oral solution contains sorbitol, sodium, sodium benzoate, and propylene glycol
This medicine contains 32.97 mg of sorbitol in each ml. Sorbitol is a source of fructose. If your doctor has told you that you (or your child) have an intolerance to some sugars, or have been diagnosed with a rare genetic disorder called hereditary fructose intolerance (HFI), in which the body is unable to break down fructose, consult your doctor before taking this medicine.
This medicine contains 2.64 mg of sodium (a major component of cooking/table salt) in each ml. This is equivalent to 3.3% of the maximum recommended daily intake of sodium for an adult.
This medicine contains 0.8 mg of sodium benzoate in each ml.
This medicine contains 9.8 mg of propylene glycol in each ml.
3. How to take Strattera
?
- Children should not take this medicine without the help of an adult.
- If you feel drowsy or nauseous when taking Strattera once a day, your doctor may change the dosing to twice a day.
- The medicine can be taken with or without food.
- The oral solution should not be mixed with food or water as this may reduce the amount taken or make the taste less pleasant.
- Taking the medicine at the same time each day can help you remember to take it.
Strattera oral solution is available in a bottle. This forms part of a pack which also includes a dosing device consisting of a 10 ml oral syringe graduated in 1 ml fractions and a press-in bottle adapter. To see the instructions on how to use the adapter and the dosing syringe, read the instructions in the user manual that is included in the pack.
How much to takeIf you are a child (over 6 years) or an adolescent:Your doctor will tell you what dose of Strattera to take based on your weight. You will normally start with a low dose before increasing it, based on your weight.
- Up to 70 kg body weight: starting with a total daily dose of 0.5 mg/kg body weight for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 1.2 mg/kg body weight per day.
- Over 70 kg body weight: starting with a total daily dose of Strattera 40 mg for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 80 mg per day. The maximum daily dose is 100 mg.
Adults
- Strattera should be started with a total daily dose of 40 mg for a minimum of 7 days. Your doctor will then decide if the dose should be increased to the usual maintenance dose of 80 mg to 100 mg per day. The maximum daily dose is 100 mg.
If you have liver problems, your doctor may recommend a lower dose.
If you take more Strattera than you shouldIn case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the National Poison Information Service on 91 562 04 20, stating the name of the medicine and the amount taken. The most commonly reported symptoms accompanying overdoses are gastrointestinal symptoms, somnolence, dizziness, tremor, and abnormal behaviour. Rarely, seizures have been reported, and very rarely, serotonin syndrome (a potentially life-threatening condition; see section 2, Warnings and precautions, Serotonin Syndrome) has been reported.
If you forget to take StratteraIf you miss a dose, take another as soon as you remember, but do not take more than the total daily dose in a 24-hour period. Do not take a double dose to make up for a forgotten dose.
If you stop taking StratteraIf you stop taking Strattera, there are usually no withdrawal effects, but the symptoms of ADHD may return. You should talk to your doctor before stopping treatment.
What your doctor will do while you are taking Strattera
Your doctor will do some tests
- before starting - to make sure Strattera is safe and will be of benefit
- after starting - at least every 6 months, although this may be more often
Tests will also be done when the dose is changed. These tests will include:
- measuring height and weight in children and adolescents
- measuring blood pressure and heart rate
- checking if you have any problems or if side effects have got worse while you are taking Strattera
Long-term treatmentStrattera does not need to be taken indefinitely. If you take Strattera for more than a year, your doctor should review your treatment to see if the medicine is still needed.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Although some people suffer from adverse effects, most consider that Strattera helps them. Your doctor will discuss these adverse effects with you.
Some adverse effects could be serious. If you experience any of the following effects, contact your doctor immediately.
Uncommon(may affect up to 1 in 100 people)
- feeling or having a very fast heart rate, abnormal heart rhythm
- thoughts or feelings of suicide
- feeling aggressive
- feeling of hostility and anger
- mood changes
- severe allergic reaction with symptoms of
- swelling of the face and throat
- difficulty breathing
- hives (small, itchy, red rashes on the skin)
- seizures
- psychotic symptoms including hallucinations (such as hearing voices or seeing things that do not exist), believing things that are not true, or being suspicious.
Children and young people under 18 years have a higher risk of suffering from adverse effects such as:
- thoughts or feelings of suicide (may affect up to 1 in 100 people)
- mood changes (may affect up to 1 in 10 people)
Adults have a lower risk(may affect up to 1 in 1,000 people) of suffering from adverse effects such as:
- seizures
- psychotic symptoms including hallucinations (such as hearing voices or seeing things that do not exist), believing things that are not true, or being suspicious.
Rare(may affect up to 1 in 1,000 people)
- liver problems
Stop treatment with Strattera and contact your doctor immediately if you experience any of the following adverse effects:
- dark urine
- yellowish skin and eyes
- pain when touching the upper right part of the abdomen, just below the ribs
- unexplained feeling of discomfort (nausea)
- fatigue
- itching
- feeling of starting with a cold
Other reported adverse effects have been the following. If any of them worsen, consult your doctor or pharmacist.
Adverse effects very common(may affect more than 1 in 10 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
These effects may disappear over time in most patients |
|
Adverse effects common(may affect up to 1 in 10 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
|
Adverse effects uncommon(may affect up to 1 in 100 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
|
Adverse effects rare(may affect up to 1 in 1,000 people) | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
|
Frequency not known | |
CHILDREN over 6 years and YOUNG PEOPLE | ADULTS |
|
Effects on GrowthWhen some children start taking Strattera, their growth (weight and height) is reduced. However, with long-term treatment, children recover the expected weight and height for their age range. Your doctor will monitor your child's height and weight. If your child is not growing or gaining weight as expected, your doctor may change the dose of Strattera or temporarily suspend treatment with Strattera.
Reporting Adverse EffectsIf you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Strattera
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and on the bottle after "EXP". The expiration date is the last day of the month indicated.
Do not use the oral solution more than 45 days after the first opening of the bottle.
This medicine does not require special storage conditions.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Strattera 4mg/ml
- The active ingredient is atomoxetine hydrochloride. Each ml of oral solution contains atomoxetine hydrochloride equivalent to 4 mg of atomoxetine.
- The other components are sodium benzoate (E211), sodium dihydrogen phosphate dihydrate, phosphoric acid, liquid sorbitol (crystallizable) E420, xylitol, artificial raspberry flavor (containing propylene glycol E1520), sucralose, sodium hydroxide, purified water.
Appearance of the product and packaging contentOral solution, 4 mg/ml (clear colorless)
Strattera oral solution is available in a bottle containing 100 ml of solution, with a child-resistant cap. The packaging also includes a dosing device consisting of a 10 ml oral dosing syringe graduated in fractions of 1 ml and a bottle adapter.
Strattera oral solution is available in a package containing a single bottle and in a multiple package with three bottles.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturerMarketing authorization holder: Lilly S.A. Avda de la Industria 30, 28108 Alcobendas, Madrid, Spain.
Manufacturer: Patheon France, 40 Boulevard de Champaret, 38300 Bourgoin-Jallieu, France.
Strattera is a registered trademark of Eli Lilly and Company Limited.
You can request more information about this medication by contacting the local representative of the marketing authorization holder:Irisfarma S.A. Avda. de la Industria 30, 28108 Alcobendas, Madrid, Spain.
This medication is authorized in the member states of the European Economic Area under the following names:Austria, Denmark, Germany, Hungary, Ireland, Italy, Malta, Norway, Portugal, Romania, Slovenia, Spain, Sweden: Strattera.
Date of the last revision of this prospectus: November 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
Stratteraatomoxetine oral solution
Instructions for UseYour step-by-step guide to using STRATTERAWHEN USING STRATTERA, carefully read and follow the step-by-step instructions.
WARNING:The adapter poses a CHOKING HAZARD as it is a small component. Do not attach the syringe to the adapter until the adapter is completely inserted into the bottle. For safe use, the adapter must be completely inserted into the bottle. Use only under adult supervision.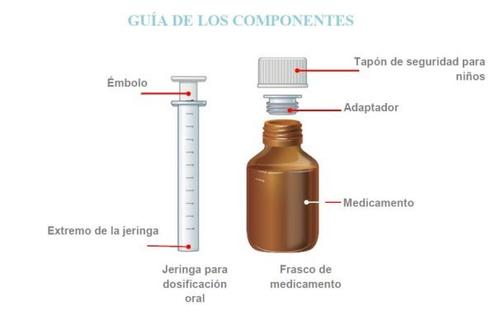 . IMPORTANTDO NOTlet your child take the medication without your help. DO NOTuse the medication if it has expired (check the expiration date on the label). DO NOTuse the medication if more than 45 days have passed since you first opened the bottle. See the Disposalsection for more information on what to do with unused medication. DO NOTwash the oral dosing syringe with soap or detergent. DO NOTput the syringe in the dishwasher. If you do, the syringe may not work as well as it should. Please see the cleaning instructions from step N to P. It is not recommended to mix STRATTERA oral solution with food or water as it may affect the taste or prevent the full dose from being taken. STRATTERA can cause eye irritation. Avoid eye contact.If the medication comes into contact with the eyes, rinse them immediately with water and consult your doctor. Wash your hands and any surfaces that may have come into contact with the medication as soon as possible.
. IMPORTANTDO NOTlet your child take the medication without your help. DO NOTuse the medication if it has expired (check the expiration date on the label). DO NOTuse the medication if more than 45 days have passed since you first opened the bottle. See the Disposalsection for more information on what to do with unused medication. DO NOTwash the oral dosing syringe with soap or detergent. DO NOTput the syringe in the dishwasher. If you do, the syringe may not work as well as it should. Please see the cleaning instructions from step N to P. It is not recommended to mix STRATTERA oral solution with food or water as it may affect the taste or prevent the full dose from being taken. STRATTERA can cause eye irritation. Avoid eye contact.If the medication comes into contact with the eyes, rinse them immediately with water and consult your doctor. Wash your hands and any surfaces that may have come into contact with the medication as soon as possible.
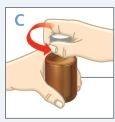
STEP 1 INITIAL BOTTLE SETUP
| Only before the first use, push the adapter until it is fully seatedon the bottle. Do not assemble the syringe to the adapter until the adapter is completely inserted into the bottle. DO NOTtwist the adapter. WARNING:The adapter poses a CHOKING HAZARD as it is a small component. For safe use, it must be completely inserted into the bottle. |
STEP 2 PREPARE
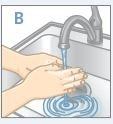
| Gather all the items you will need:
|
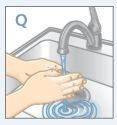
| Wash your hands with water and soap |
Write your child's dose here: ____ ml Make sure it is the exact dose prescribed by the doctor. If your child's dose is10 ml OR LESS, you will use the syringe 1 time. If your child's dose isMORE THAN 10 mlbut NOT more than 20 ml, you will use the same syringe 2 times. If your child's dose isMORE THAN 20 ml, you will use the same syringe 3 times. STEP 3 OBTAINING THE DOSE
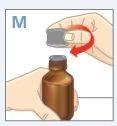
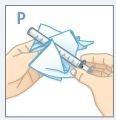
| Press the cap down while turning it counterclockwise. Remove the cap from the bottle. |
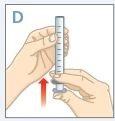
| Push the plunger all the way down to the end of the syringe. |
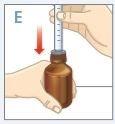
| Insert the tip of the syringe into the adapter. Make sure the tip of the syringe is fully seated in the adapter. |
| Turn the bottle and syringe upside down. |
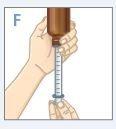
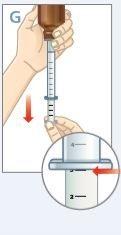
| Hold the bottle and syringe with 1 hand. With the other hand, pull the plunger down to withdraw the appropriate amount of medication into the syringe. Check that the dose in the syringe is correct. |
| Turn the bottle upright and place it on a flat surface. Check that there are no air bubbles in the syringe. If there are, return the medication to the bottle and repeat steps D to H. An air bubble can result in an incorrect dose. |
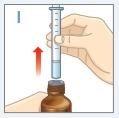
| Remove the syringe from the bottle. DO NOTtouch the plunger. |
STEP 4 ADMINISTERING THE MEDICATION
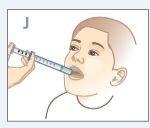
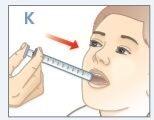
| Place the tip of the syringe on one side of your child's mouth. Tell your child not to bite the syringe. |
| Push the plunger down until ALLthe medication is in your child's mouth. DO NOTdirect the medication towards the back of your child's throat. Make sure your child swallows all the medication. |
| If your child's dose isGREATER THAN 10 ml, repeat from step D to K to give the remaining dose. Use the same syringe. See the dosing charton the other side of this document. Make sure you administer the exact dose prescribed by your child's doctor. |
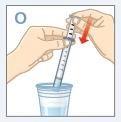
STEP5CLEANINGFACE 1
| Tighten the bottle cap firmly. DO NOTremove the adapter. The cap will adapt. |
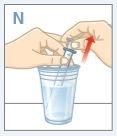
| Fill a glass with clean water. DO NOTwash the oral dosing syringe with soap or detergent. DO NOTremove the plunger from the oral dosing syringe. Place the tip of the syringe in the water. Pull the plunger up to fill it with water. |
| Push the plunger down and empty the water into the glass or sink. |
| Remove excess water from the syringe. Dry the syringe with a paper towel. |
| Wash your hands and your child's hands with soap and water. DO NOTtouch your eyes after coming into contact with STRATTERA. STRATTERA can irritate your eyes. |
See the other side of this document for answers to frequently asked questions, to check the dosing chart, and for other important information.
CARA 2
DOSING CHARTFORDOSESOFMORETHAN10mlUse this chart to calculate the dose to administer to your child. Find the correct dose in the first column. Consult with your doctor or pharmacist on how to administer the correct dose to your child.
If this is your child's dose | Put this amount of STRATTERA in the syringe, for the first time | Put this amount of STRATTERA in the syringe, a second time | Put this amount of STRATTERA in the syringe, a third time |
11 ml | 10 ml | 1 ml | 0 |
12 ml | 10 ml | 2 ml | 0 |
13 ml | 10 ml | 3 ml | 0 |
14 ml | 10 ml | 4 ml | 0 |
15 ml | 10 ml | 5 ml | 0 |
16 ml | 10 ml | 6 ml | 0 |
17 ml | 10 ml | 7 ml | 0 |
18 ml | 10 ml | 8 ml | 0 |
19 ml | 10 ml | 9 ml | 0 |
20 ml | 10 ml | 10 ml | 0 |
21 ml | 10 ml | 10 ml | 1 ml |
22 ml | 10 ml | 10 ml | 2 ml |
23 ml | 10 ml | 10 ml | 3 ml |
24 ml | 10 ml | 10 ml | 4 ml |
25 ml | 10 ml | 10 ml | 5 ml |
Frequently Asked QuestionsQ.What happens if there is an air bubble in the oral dosing syringe?A.DO NOTadminister the medication to your child. Air bubbles can make the dose incorrect. Empty the medication back into the bottle and repeat from step D to H. Q.What happens if there is too much medication in the syringe?A.Keep the tip of the syringe in the bottle. Hold the bottle upside down. Push the plunger down until the correct dose is in the syringe. Q.What happens if there is not enough medication in the syringe?A.Keep the tip of the syringe in the bottle. Hold the bottle upside down. Pull the plunger down until the correct dose is in the syringe. Q.What do I do if the medication comes into contact with my eyes or my child's eyes?A.Rinse your eyes immediately with water and consult your doctor. As soon as possible, wash your hands and any surface that may have come into contact with the medication. Q.How do I travel with this medication?A. Make sure you have enough STRATTERA for the entire trip. Keep the medication in a safe place at room temperature. Q.Can I mix STRATTERA with food or water before giving it to my child?A. It is not recommended to mix STRATTERA oral solution with food or water. It may affect the taste of the oral solution or prevent the full dose from being taken. Your child may drink a glass of water after taking the entire dose of the medication. StorageThis medication does not require special storage conditions. Keep the bottle and syringe out of sight and reach of children. DisposalDo not throw away medications directly down the drain or into the trash. Consult your pharmacist on how to dispose of unused medications. These measures will help protect the environment.
- Country of registration
- Average pharmacy price106.15 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STRATTERA 4 mg/ml ORAL SOLUTIONDosage form: CAPSULE, 10 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription requiredDosage form: CAPSULE, 100 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription requiredDosage form: CAPSULE, 18 mgActive substance: atomoxetineManufacturer: Laboratorios Rubio S.A.Prescription required
Online doctors for STRATTERA 4 mg/ml ORAL SOLUTION
Discuss questions about STRATTERA 4 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions