
STESOLID 10 mg rectal solution

How to use STESOLID 10 mg rectal solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Stesolid 10 mg Rectal Solution
diazepam
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Stesolid 10 mg and what is it used for
- What you need to know before you use Stesolid 10 mg
- How to use Stesolid 10 mg
- Possible side effects
- Storing Stesolid 10 mg
- Contents of the pack and other information
1. What is Stesolid 10 mg and what is it used for
Diazepam belongs to a group of medicines called benzodiazepines. It is used to treat febrile convulsions in children over 1 year old (approximately 10 kg) and epileptic convulsions; anxiety, distress or tension and as a sedative in minor surgery, diagnostic interventions and endoscopic procedures.
2. What you need to know before you use Stesolid 10 mg
Do not use Stesolid 10 mg
- if you are allergic (hypersensitive) to the active substance or any of the other ingredients of this medicine (see section 6).
- if you are allergic (hypersensitive) to any of the medicines known as "benzodiazepines".
- if you have a muscle disease called "myasthenia gravis".
- if you have respiratory problems such as sleep apnea syndrome (brief stops in breathing during sleep) or severe respiratory failure.
- if you have severe liver disease (severe hepatic insufficiency).
- if you have closed-angle glaucoma (an eye disease that causes increased pressure inside the eyeball).
- if you are dependent on other substances, including alcohol. An exception to this is the treatment of acute withdrawal reactions.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Stesolid
- if you have used the medicine continuously for a long period of time, as it may cause dependence. It is not recommended for chronic use as a daily anticonvulsant due to the fact that after continuous use, a certain degree of loss of efficacy may be detected.
- if you are epileptic. You should be aware that some cases of increased convulsions have been reported in epileptic patients treated with diazepam.
- if you have porphyria, as diazepam may cause a relapse of the disease.
- if you have any liver or kidney disorder. Your doctor will decide whether you should use a lower dose or not take the medicine.
- if you are an elderly patient. Due to the muscle relaxant effect, there is a risk of falls and consequently fractures, especially when getting up at night. In these patients, a dose of 5 mg is recommended, as indicated in section 3 of this leaflet.
If after taking the medicine you experience reactions such as: restlessness, agitation, irritability, aggression, delirium, fits of rage, nightmares, hallucinations, psychosis, inappropriate behavior, and other adverse effects on behavior; inform your doctor immediately. These reactions are more frequent in children and elderly patients.
After taking this medicine, you should make sure you can sleep uninterrupted for 7-8 hours. Otherwise, although this is rare, you may not remember part of what happened while you were awake.
If you have had problems with drug or alcohol dependence, you should not use this medicine unless your doctor formally indicates it.
Diazepam may alter the values of the following analytical determinations:
- Blood: increase (biological) of cholesterol, estradiol, phenytoin, prolactin, and testosterone. Reduction (biological) of thyroxine.
- Urine: increase (analytical interference) of 5-hydroxyindoleacetic acid. Reduction (analytical interference) of glucose.
Children and Adolescents
Due to the lack of safety and efficacy studies, the use of Stesolid is not recommended in children under 1 year of age (less than 10 kg), unless the doctor indicates otherwise.
Other Medicines and Stesolid
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
The administration of Stesolid together with other medicines that act on the central nervous system, such as those used to treat insomnia, anxiety, depression, severe pain, psychiatric problems, epilepsy, and allergy, as well as some anesthetics, may make the action of the medicine too strong. If you are taking any medication for the treatment of any of these diseases, you should inform your doctor.
Keep in mind that these instructions may also apply to medications that have been taken before or may be taken later.
Tell your doctor if you are taking or have taken any of these medicines:
- amitriptyline, fluoxetine, fluvoxamine, sertraline, lithium, haloperidol (medicines used to treat depression and other psychiatric disorders),
- clozapine (medicine for treating schizophrenia)
- amprenavir, atazanavir, ritonavir (medicines used to treat HIV/AIDS infection),
- fluconazole, itraconazole, ketoconazole (medicines used to treat fungal infections),
- barbiturates (medicines used as sedatives or anesthetics),
- propranolol, metoprolol (medicines for treating high blood pressure),
- buprenorphine, fentanyl (medicines for pain),
- central-acting muscle relaxants such as baclofen, chlorzoxazone, tizanidine, methocarbamol,
- cimetidine, omeprazole (medicines to prevent or treat stomach ulcers),
- digitalis medicines (medicines for the heart),
- ethinyl estradiol, mestranol (oral contraceptives),
- phenytoin, phenobarbital, carbamazepine, valproic acid (medicines for treating epilepsy)
- rifampicin, isoniazid (used to treat tuberculosis),
- propofol (anesthetic),
- clarithromycin, erythromycin, roxithromycin (antibiotics)
- disulfiram (medicine used to help treat chronic alcoholism)
- levodopa (medicine used in the treatment of Parkinson's disease)
- ethanol,
- caffeine, theophylline
- ginkgo, St. John's wort (hypericum perforatum), valerian.
Using Stesolid 10 mg with Food and Drinks
You should not drink alcoholic beverages while using this medicine.
Pregnancy and Breastfeeding
Consult your doctor or pharmacist before using any medicine.
You should not use this medicine during pregnancy unless your doctor considers it essential.
This medicine is excreted in breast milk, so if necessary, breastfeeding should be suspended.
Driving and Using Machines
Stesolid may alter your ability to drive or operate machinery as it can cause drowsiness, reduce your attention, or reduce your reaction capacity. The appearance of these effects is more likely at the start of treatment or when the dose is increased. Do not drive or use machines if you experience any of these effects. The concomitant administration with alcoholic beverages can enhance these effects.
Stesolid contains benzoic acid (E-210), sodium benzoate (E-211), benzyl alcohol, propylene glycol (E-1520), and ethanol
This medicine contains 2.5 mg of benzoic acid (equivalent to 1 mg/ml) and 122.5 mg of sodium benzoate (equivalent to 49 mg/ml) in each single-dose container. Benzoic acid and sodium benzoate may cause local irritation. Benzoic acid and sodium benzoate may increase the risk of jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks of age).
This medicine contains 37.5 mg of benzyl alcohol in each single-dose container (equivalent to 15 mg/ml). Benzyl alcohol may cause allergic reactions and moderate local irritation.
This medicine contains 1000 mg of propylene glycol in each single-dose container (equivalent to 400 mg/ml). Propylene glycol may cause skin irritation. Do not use this medicine in babies under 4 weeks with open wounds or large areas of damaged skin (such as burns) without consulting your doctor or pharmacist first.
This medicine contains 250 mg of ethanol in each single-dose container (equivalent to 100 mg/ml). It may cause a burning sensation on damaged skin. In newborns (premature and full-term babies), high concentrations of ethanol can cause severe local reactions and systemic toxicity due to significant absorption through immature skin.
Ask your doctor or pharmacist for advice if your child is under 5 years old, if you have liver or kidney disease, or if you are pregnant or breastfeeding. This is because the excipients can cause adverse effects. Your doctor may need to adjust the dose if you or your child are using other medicines that contain benzyl alcohol, propylene glycol, or alcohol.
3. How to Use Stesolid 10 mg
Follow the administration instructions of Stesolid 10 mg indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Stesolid 10 mg is administered rectally.
To ensure the correct administration of this medicine, the following RULES FOR THE CORRECT ADMINISTRATION OF STESOLID will be followed:
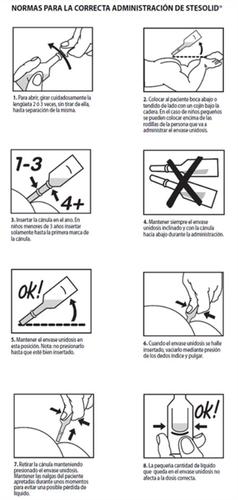
The recommended dose is:
Children
- 5 mg for children weighing between 10-15 kg (approximately 1 to 3 years). In this case, it is recommended to use a single-dose container of the 5 mg presentation (Stesolid 5 mg rectal solution).
- 10 mg for children over 16 kg in weight (approximately 3 years).
Your child should not receive more than one dose in a single day if they are under 5 years old.
Stesolid 10 mg is not indicated for children under 1 year of age.
Adults
- 10 mg in most cases.
Elderly Patients and Patients with Hepatic Insufficiency
The recommended dose in elderly patients and patients with hepatic insufficiency is 5 mg. In this case, it is recommended to use a single-dose container of the 5 mg presentation (Stesolid 5 mg rectal solution).
Patient with Renal Insufficiency
Although it has not been demonstrated that doses need to be reduced if you have kidney disease, caution is advised. If this is your case, consult your doctor before using this medicine.
If you think the effect of Stesolid is too strong or too weak, inform your doctor or pharmacist.
If you use more Stesolid 10 mg than you should
You may experience symptoms ranging from drowsiness to coma. However, given the route of administration, the possibility of overdose is unlikely.
If this occurs, consult your doctor or pharmacist immediately or go to the nearest hospital and bring this leaflet.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, Telephone 91 562 04 20.
If you forget to use Stesolid 10 mg
Do not use a double dose to make up for forgotten doses.
If you stop using Stesolid 10 mg
Your doctor will indicate the duration of your treatment with Stesolid. Do not stop treatment before or abruptly, as your problem may reappear or you may experience other symptoms.
If you stop using the medicine abruptly, you may experience the following symptoms: insomnia, headache or muscle pain, anxiety, tension, restlessness, confusion, and irritability. Treatment should be discontinued gradually, following the instructions received from your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, Stesolid 10 mg can cause side effects, although not everybody gets them.
Normally, with medicines that contain diazepam, at the beginning of treatment, side effects such as drowsiness, apathy, decreased alertness, confusion, fatigue, headache, dizziness, muscle weakness, lack of coordination, or double vision have been detected. These phenomena usually disappear with continued treatment.
Occasionally, other side effects such as gastrointestinal disorders, skin reactions, or changes in sexual desire have been reported.
In general, after the administration of diazepam, the following side effects have been described:
Very common (may affect more than 1 in 10 patients)
Common (may affect between 1 and 10 in 100 patients)
Uncommon (may affect between 1 and 10 in 1,000 patients)
Rare (may affect between 1 and 10 in 10,000 patients)
Very rare (may affect less than 1 in 10,000 patients)
Blood and lymphatic system disorders
Rare: neutropenia (decrease in the number of neutrophils), with prolonged administration of diazepam.
Very rare: anemia, pancytopenia (decrease in all blood cell elements), decreased platelet count.
Immune system disorders
Very rare: allergies
Endocrine disorders
Very rare: Cases of milk secretion from the nipple have been described in women with normal serum prolactin values and increased breast size in men who regularly took diazepam.
Benzodiazepines may decrease body temperature, probably in a dose-dependent manner; this effect has been observed in neonates of mothers who took benzodiazepines in the last stages of pregnancy.
Psychiatric disorders
Rare: depression, decreased sexual desire, paradoxical excitement that may include: hostility, aggression, and disinhibition, anxiety, hallucinations, insomnia, sleep disturbances. Mild mental disorders: memory loss, slowed reaction time.
Very rare: difficulty remembering some things.
Nervous system disorders
Very common: drowsiness.
Common: headache, lack of muscle coordination, dizziness, confusion.
Uncommon: hyperactivity, speech disorders, tremors.
Rare: rebound convulsions.
Eye disorders
Rare: blurred vision, double vision, involuntary eye movements.
Diazepam has some anticholinergic activity, so it may cause pupil dilation and therefore exacerbate narrow-angle glaucoma.
Ear and labyrinth disorders
Uncommon: vertigo.
Cardiac disorders (only described when administered intravenously)
Uncommon: alteration of heart rhythm and inflammation of veins with thrombus formation.
In rapid intravenous administration
Uncommon: thrombus formation in the veins, vein inflammation, local irritation, swelling.
Rare: severe hypotension, decreased heart rate, circulatory collapse.
Vascular disorders
Very rare: bullosus vasculitis (purple-colored skin rash due to alteration of small vessels).
Respiratory disorders
Common: respiratory depression.
Gastrointestinal disorders
Uncommon: gastrointestinal disorders, nausea, constipation, alterations in salivation.
Hepatobiliary disorders
Very rare: yellowing of the skin, elevated liver enzymes.
Skin and subcutaneous tissue disorders
Uncommon: rash and urticaria, sweating, local irritation after rectal administration.
Very rare: A clinical picture has been described that occurs with fever, rash, and blood alterations (Sweet's syndrome).
Musculoskeletal disorders
Very rare: In patients with low sodium levels in the blood, muscle pain and weakness can occur, which can be severe.
Renal and urinary disorders
Uncommon: urinary retention, incontinence
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Stesolid 10 mg
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the container after CAD. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Stesolid 10 mg
- The active ingredient is diazepam. Each 2.5 ml single-dose container of rectal solution contains 10 mg of diazepam.
- The other components (excipients) are: benzoic acid (E-210), sodium benzoate (E-211), propylene glycol (E-1520), ethanol, benzyl alcohol, and purified water.
Appearance of the Product and Package Contents
Stesolid 10 mg is a practically colorless transparent solution presented in single-dose containers for rectal administration. Each single-dose container contains 2.5 ml of rectal solution.
It is presented in boxes containing 2 and 5 single-dose containers.
Marketing Authorization Holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma, S.A.
Maximo Agirre Kalea, 14
48940 Leioa (Bizkaia)
Spain
Or
Faes Farma, S.A.
Bizkaia Science and Technology Park
Ibaizabal Bidea, Building 901
48160 Derio (Bizkaia)
Spain
Date of the Last Revision of this Leaflet: October 2024
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price10.93 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STESOLID 10 mg rectal solutionDosage form: TABLET, 10 mgActive substance: diazepamManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 2.5 mgActive substance: diazepamManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 5 mgActive substance: diazepamManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for STESOLID 10 mg rectal solution
Discuss questions about STESOLID 10 mg rectal solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











