
SILVEDERMA 10 mg/g CREAM

How to use SILVEDERMA 10 mg/g CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Silvederma 10 mg/g Cream
Silver Sulfadiazine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack:
- What Silvederma 10 mg/g Cream is and what it is used for
- What you need to know before you use Silvederma 10 mg/g Cream
- How to use Silvederma 10 mg/g Cream
- Possible side effects
- Storage of Silvederma 10 mg/g Cream
- Contents of the pack and other information
1. What Silvederma 10 mg/g Cream is and what it is used for
This medicine contains silver sulfadiazine, which is an antibiotic belonging to the sulfonamide group (a type of medicine used to treat infections).
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential that you follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste.
This medicine is indicated for the treatment and prevention of infections in second- and third-degree burns, as well as in varicose and pressure ulcers.
2. What you need to know before you use Silvederma 10 mg/g Cream
Do not use Silvederma 10 mg/g Cream:
- If you are allergic (hypersensitive) to silver sulfadiazine, sulfonamides (the group to which this medicine belongs), or any of the other components of this medicine (listed in section 6).
- In the case of large surface lesions in newborn, premature babies, during the last days of pregnancy, or during breastfeeding.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Silvederma 10 mg/g Cream.
- There have been reports of skin eruptions that can be life-threatening (Stevens-Johnson syndrome and toxic epidermal necrolysis) with the use of Silvederma 10 mg/g Cream. Initially, they appear as red, circular spots or patches, often with a central blister.
- Other additional signs that may appear are sores in the mouth, throat, nose, genitals, and conjunctivitis (swollen and red eyes).
- These life-threatening skin eruptions are often accompanied by flu-like symptoms. The eruption can progress to the formation of widespread blisters or skin peeling.
- The period of highest risk for the occurrence of severe skin reactions is during the first few weeks of treatment.
- If you have developed Stevens-Johnson syndrome or toxic epidermal necrolysis with the use of Silvederma 10 mg/g Cream, you should not use Silvederma cream again at any time.
- If you develop a rash or these skin symptoms, stop using Silvederma 10 mg/g Cream, go to a doctor immediately, and inform them that you are using this medicine.
Be careful with Silvederma 10 mg/g Cream:
- If you have liver or kidney disease, you should avoid applying the cream to large or open lesions, especially ulcers.
- If you have a reduced white blood cell count, your doctor will perform control counts.
- If you have a deficiency of the enzyme glucose-6-phosphate dehydrogenase.
- You should not expose the treated area with this medicine to direct sunlight, as it may cause skin discoloration, as well as a grayish coloration of the cream.
Using Silvederma 10 mg/g Cream with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You should not use this medicine during the last days of pregnancy or during breastfeeding.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
Silvederma contains propylene glycol, cetoestearilic alcohol, and methyl parahydroxybenzoate (E218)
This medicine may cause skin irritation because it contains propylene glycol.
This medicine may cause local skin reactions (such as contact dermatitis) because it contains cetoestearilic alcohol.
This medicine may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate (E-218).
3. How to use Silvederma 10 mg/g Cream
Follow exactly the administration instructions of this medicine as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
It is administered topically via the skin.
Initially, you should wash and clean the wound. Then, with a sterile spatula or with your hand covered with a sterile glove, you should apply a 3 mm thick layer over the affected area, covering it with a suitable dressing.
Normally, the dressing should be renewed 1-2 times a day, and it may be renewed every 4-6 hours in the case of highly contaminated wounds.
At each dressing change and reapplication of the medicine, you should first remove the remains of the previous application, carefully washing the wound with warm boiled water or isotonic saline solution.
Each container should be used for a single patient.
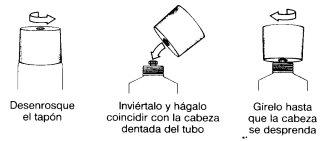
Instructions for the correct administration of the preparation:
The tube has a seal that is opened by following the instructions in the drawing:
If you use more Silvederma 10 mg/g Cream than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested or used.
If you forget to use Silvederma 10 mg/g Cream
If you forget to apply a dose, apply it as soon as you remember. Do not take a double dose to make up for forgotten doses.
If you have any doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very rare side effects (may affect up to 1 in 10,000 people):
- Life-threatening skin eruptions (Stevens-Johnson syndrome, toxic epidermal necrolysis) (see section 2).
- Side effects with an unknown frequency (cannot be estimated from the available data). If you notice any of the following reactions, inform your doctor immediately: Allergic reactions.
- Skin reactions such as a burning sensation or pain.
- Gray skin discoloration in the application area due to sun exposure.
- Decreased white blood cell count (leukopenia).
- Increased osmolality in serum.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency (AEMPS) https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Silvederma 10 mg/g Cream
Keep this medicine out of the sight and reach of children.
No special storage conditions are required. Store in the original packaging to protect it from light.
Do not use this medicine after the expiry date stated on the packaging. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and other information
Composition of Silvederma 10 mg/g Cream:
- The active ingredient is silver sulfadiazine. Each gram of cream contains 10 milligrams of silver sulfadiazine.
- The other ingredients are: cetoestearilic alcohol, isopropyl myristate, white petrolatum, polyoxyl 40 stearate, sorbitan oleate, propylene glycol (E-1520), methyl parahydroxybenzoate (E-218), and purified water.
Appearance of the product and contents of the pack
Silvederma 10 mg/g Cream is a white or off-white cream, presented in tubes of 50 grams or 100 grams, and in a jar of 500 grams of cream. It is packaged in a cardboard box.
Marketing authorization holder and manufacturer:
Laboratorio ALDO-UNIÓN, S.L.
Calle Baronesa de Maldá, 73
08950 Esplugues de Llobregat
BARCELONA – SPAIN
This leaflet was approved in May 2007
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SILVEDERMA 10 mg/g CREAMDosage form: CREAM, 10 mg silver sulfadiazine/gActive substance: silver sulfadiazineManufacturer: Alliance Pharma (Ireland) LimitedPrescription requiredDosage form: TOPICAL SOLUTION, 10 mg/mlActive substance: silver sulfadiazineManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: CREAM, -Active substance: silver sulfadiazine, combinationsManufacturer: Alliance Pharma (Ireland) LimitedPrescription required
Online doctors for SILVEDERMA 10 mg/g CREAM
Discuss questions about SILVEDERMA 10 mg/g CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








