
SICCAFLUID 2.5 mg/g OPHTHALMIC GEL SINGLE-DOSE

How to use SICCAFLUID 2.5 mg/g OPHTHALMIC GEL SINGLE-DOSE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SICCAFLUID 2.5 mg/g OPHTHALMIC GEL IN UNIT DOSE
Carbomer 974 P
Read this package leaflet carefully because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If your symptoms worsen or persist, you should consult your doctor.
- If you think any of the side effects that you are suffering from is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Contents of the Package Leaflet
- What is Siccafluid and what is it used for
- Before using Siccafluid
- How to use Siccafluid
- Possible side effects
- Storage of Siccafluid
- Further information
1. What is Siccafluid and what is it used for
Siccafluid is a tear substitute and contains a lubricant called Carbomer 974P.
It is an ophthalmic gel used for the relief of symptoms of dry eyes (such as inflammation, burning, irritation, or dryness) caused when the eyes do not produce enough tears.
2. Before using SICCAFLUID
Do not use Siccafluid
•If you are allergic (hypersensitive) to carbomer or any of the other components of Siccafluid listed in section 6, "What contains Siccafluid".
Be careful with Siccafluid
•If your condition worsens or does not improveafter starting treatment with Siccafluid, contact your doctor.
•DO NOT INJECT, DO NOT INGEST.
Children and adolescents up to 18 years of age
The safety and efficacy of SICCAFLUID in children and adolescents at the recommended posology for adults have been established based on clinical experience, but no clinical studies are available.
Use of other medications
Tell your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
If you need to use any other ophthalmic medication during treatment with Siccafluid: first use the other ophthalmic medication and wait 15 minutes before using Siccafluid.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
If you are pregnant or breastfeeding, contact your doctor to consult before starting the use of Siccafluid.
He or she will decide if you can use Siccafluid.
Driving and using machines
Your vision may become blurry for a short time after using Siccafluid.
You should not drive or use machinery until your vision returns to normal.
3. How to use Siccafluid
If you have been recommended Siccafluid for use, follow exactly the administration instructions indicated by your doctor. Consult your doctor or pharmacist if you have doubts.
The doseusuallyis 1 drop administered in the affected eye or eyesup to4 times a day.
Children and adolescents up to 18 years of age
The safety and efficacy of SICCAFLUID in children and adolescents at the recommended posology for adults have been established based on clinical experience, but no clinical studies are available.
How to use:
Wash your hands before opening the unit dose container.
Check that the gel is at the tip of the container. To open the container, turn until the tab on the top breaks.
Tilt your head back and look up.
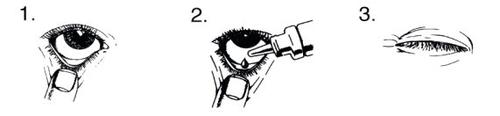
- Gently pull down the lower eyelid of the affected eye until a small "pouch" forms.
- Turn the container upside down. Press until a drop falls into the "pouch".
- Release the lower eyelid and blink several times.
- Repeat steps 1 to 3 in the other eye if it also needs to be treated.
To help prevent infection, do not touch the eye, surrounding areas, or any other area with the tip of the container.
Discard the unit dose container after use. Do not save it for later use.
If you use more Siccafluid than you should
Using more drops of Siccafluid than you should does not cause any harm.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use Siccafluid
Do not use a double dose to make up for the forgotten dose. Apply the next dose according to the frequency you have established.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Siccafluid can cause side effects, although not everyone gets them.
Contactyour doctor if:
•Your symptoms worsen or do not improve after starting treatment with Siccafluid.
If you experience any of the following side effects after applying the ophthalmic gel to the eye, talk to your doctor if it concerns you:
•Transient blurred vision
•Mild itching or burning sensation in the eye.
The side effects mentioned may occur, but the number of people likely to be affected may vary.
Reporting side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect that does not appear in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Siccafluid
Keep out of the reach and sight of children.
Do not use Siccafluid 2.5 mg/g ophthalmic gel after the expiration date that appears on the container and on the box after CAD. The expiration date is the last day of the month indicated.
Do not store above 25°C. Keep the unit dose containers in the original packaging to protect them from light.
Discard the unit dose container with the remaining solution immediately after use.
Do not save for later use.
Medications should not be thrown away through wastewater or household waste. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need.In this way, you will help protect the environment.
6. Further information
Composition of Siccafluid
- The active ingredient is Carbomer 974 P 2.5mg/g.
- The other ingredients are sorbitol, lysine monohydrate, sodium acetate trihydrate, polyvinyl alcohol, and water for injection.
Appearance of the product and container contents
Siccafluid is a slightly yellowish opalescent gel in unit dose containers.
Each unit dose container contains 0.5 g of gel.
Each box contains 10, 20, 30, or 60 unit dose containers (not all presentations may be marketed).
Marketing authorization holder
LABORATORIOS THEA S.A.
C/ Enric Granados, nº 86-88, 2ª planta, 08008 Barcelona
Manufacturer
LABORATOIRES UNITHER
1 RUE DE L’ARQUERIE 50200 COUTANCES
FRANCE
OR
LABORATOIRES UNITHER
INDUSTRIAL AREA NORTH
151, RUE ANDRÉ DUROUCHEZ – BP 28028
80084 AMIENS CEDEX-2
FRANCE
This medication is authorized in the member states of the European Economic Area under the following names:
France, Spain, Italy, and Portugal: ……………………………….. SICCAFLUID
Netherlands …………………………………………………… SICCAFLUID UNIDOSE
Iceland and Norway …………………………………………….. OFTAGEL
This package leaflet was approved in March 2015
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price7.02 EUR
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SICCAFLUID 2.5 mg/g OPHTHALMIC GEL SINGLE-DOSEDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for SICCAFLUID 2.5 mg/g OPHTHALMIC GEL SINGLE-DOSE
Discuss questions about SICCAFLUID 2.5 mg/g OPHTHALMIC GEL SINGLE-DOSE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















