
SEPTANASAL 1 mg/ml + 50 mg/ml NASAL SPRAY SOLUTION

How to use SEPTANASAL 1 mg/ml + 50 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is SeptaNasal and what is it used for
- What you need to know before you start using SeptaNasal
- How to use SeptaNasal
- Possible side effects
- Storage of SeptaNasal
- Contents of the pack and further information
- Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Introduction
Package Leaflet: Information for the User
SeptaNasal 1 mg/ml + 50 mg/ml nasal spray solution
xylometazoline hydrochloride / dexpanthenol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your doctor or pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the package leaflet
- What is SeptaNasal and what is it used for
- What you need to know before you start using SeptaNasal
- How to use SeptaNasal
- Possible side effects
- Storage of SeptaNasal
- Contents of the pack and further information
1. What is SeptaNasal and what is it used for
This medicine contains xylometazoline hydrochloride and dexpanthenol.
Xylometazoline hydrochloride causes rapid contraction of the blood vessels of the nasal mucosa and reduces inflammation of the nasal mucosa and mucous secretion. This reduces the feeling of nasal congestion. Dexpanthenol is a derivative of pantothenic acid, which promotes healing and protects the nasal mucosa.
SeptaNasal is used for local and temporary relief of nasal congestion and conditions of the nasal mucosa that are accompanied by inflammation and/or small wounds, with or without crust formation in adults and adolescents over 12 years of age.
2. What you need to know before you start using SeptaNasal
Do not use SeptaNasal
- if you or your child are allergic to xylometazoline hydrochloride, dexpanthenol, or any of the other components of this medicine (listed in section 6).
- if you have recently undergone an operation on the head (if you have undergone any cranial, transnasal, or transoral surgical intervention).
- if you have high eye pressure, especially if you have narrow-angle glaucoma.
- if you or your child have dry inflammation of the nasal mucosa (dry rhinitis).
- if you are being treated with monoamine oxidase inhibitors (MAOIs) or if you have used MAOIs in the 2 weeks prior to starting treatment with this medicine.
- if you are very prone to inflammation of the blood vessels in your nose.
- if you or your child have undergone surgical removal of the pituitary gland or other surgery that exposes the meninges (the brain lining).
This medicine must not be used in children under 12 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine:
- you are being treated with antidepressant medications, monoamine oxidase inhibitors (MAOIs), phenothiazine (a tranquilizer), methyldopa (to lower blood pressure), or other medications that may increase blood pressure.
- you have high intraocular pressure (glaucoma), especially if you have narrow-angle glaucoma.
- you have a severe cardiovascular or circulatory disease (e.g., coronary heart disease, high blood pressure, or long QT syndrome).
- you have ever experienced insomnia or vertigo when treated with other sympathomimetic medications, such as those used to treat heart disease, low blood pressure, or asthma.
- you have a metabolic disorder (e.g., diabetes, porphyria, or increased thyroid activity with increased sweating, body temperature, or heart rate).
- you have a tumor of the adrenal gland (pheochromocytoma).
- you have prostate hypertrophy.
In any of the above cases, consult your doctor or pharmacist before starting to use this medicine.
Rarely, xylometazoline may increase nasal congestion instead of reducing it; this is because the effects of xylometazoline are temporary, and prolonged use may result in a rebound effect with vasodilation, congestion, and rhinitis.
Insomnia may rarely occur after using the medicine. If this happens to you, avoid using it in the late afternoon or evening.
To avoid infections, the medicine should not be used by more than one person, and the applicator should be cleaned after each use with a clean, damp cloth.
Children
The use of SeptaNasal is only indicated in children over 12 years of age.
Use in people over 65 years of age:
Consult your doctor or pharmacist, as older people are more sensitive to the effects of this medicine.
Other medicines and SeptaNasal
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Using this medicine at the same time as certain medications for depression (monoamine oxidase inhibitors of the tranylcypromine type or tricyclic antidepressants), as well as medications that increase blood pressure, could cause an increase in blood pressure due to the cardiovascular effects of these substances.
Using this medicine at the same time as other medications for flu or cough and cold that contain sympathomimetics (medications used to treat nasal congestion, such as pseudoephedrine, ephedrine, phenylephrine, oxymetazoline, xylometazoline, tramazoline, naphazoline, or tuaminoheptane) could increase adverse reactions in the cardiovascular and central nervous systems.
Medications to lower blood pressure (e.g., methyldopa) should not be used with xylometazoline due to its hypertensive effect.
Consult a doctor before using this medicine if you or your child use any of the above medications.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
This medicine should not be used during pregnancy due to the lack of safety data in pregnant women.
This medicine should not be used during breastfeeding, as it is unknown whether xylometazoline hydrochloride is excreted in breast milk.
Driving and using machines
This medicine is not expected to affect your ability to drive or use machines if used as recommended. If you experience drowsiness or dizziness while using this medicine, do not drive or operate hazardous tools or machines.
3. How to use SeptaNasal
Follow exactly the administration instructions of this medicine contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Adults and adolescents from 12 years of age
The recommended dose is one spray in each nostril up to 3 times a day if necessary.
Do not use this medicine for more than 3 days, unless your doctor has given you different instructions.
It can only be used again after a period of several days. Do not exceed the recommended dose.
Method of administration
First, remove the protective cap from the spray.
Before the first use or if the spray has not been used for a prolonged period, press the spray head 5 times until a fine spray appears.
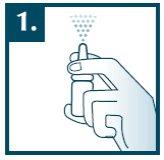
Insert the tip of the spray as straight as possible into one nostril and press the spray head once. Inhale gently through the nose while spraying the product. If necessary, repeat the procedure for the other nostril. After each use, clean the tip of the spray with a paper towel and replace the protective cap on the spray.
Prolonged or excessive use of this medicine could cause chronic inflammation and also thinning (damage) of the nasal mucosa.
Patients with high intraocular pressure (glaucoma), especially narrow-angle glaucoma, should consult a doctor before using this medicine.
Use in children and adolescents
SeptaNasal should not be administered to children under 12 years of age.
If you use more SeptaNasal than you should
- If you use more medicine than you should or if you accidentally ingest large amounts, the following side effects may occur: pupil constriction (miosis), pupil dilation (mydriasis), fever, sweating, paleness of the skin, blue discoloration of the lips (cyanosis), nausea, convulsions, heart and vascular disorders (increased heart rate, slow heart rate, arrhythmias, circulatory failure, cardiac arrest, high blood pressure), respiratory disorders (pulmonary edema, respiratory problems), and mental disorders.
You may also experience drowsiness, decreased body temperature, heart rate, and blood pressure, respiratory arrest, and coma.
If you notice any of these symptoms, inform your doctor immediately. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use SeptaNasal
Do not use a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Uncommon(may affect up to 1 in 100 people):
- hypersensitivity reaction, such as skin and mucous membrane inflammation, rash, itching.
- nasal bleeding.
Rare(may affect up to 1 in 1,000 people):
- palpitations (perception of heartbeats), tachycardia (rapid heartbeats), hypertension (high blood pressure).
Very rare(may affect up to 1 in 10,000 people):
- restlessness, difficulty sleeping, hallucinations (especially in children).
- fatigue (drowsiness, sedation), headache, seizures (especially in children).
- heart rhythm disorders (arrhythmias).
- inflammation of the nasal mucosa (after stopping treatment).
Frequency not known(cannot be estimated from the available data):
- burning and dryness of the nasal mucosa, sneezing.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Surveillance System for Human Use: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SeptaNasal
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date is the last day of the month shown.
This medicine does not require special storage conditions.
After the first opening of the container, the product should be used within 12 months.
Medicines should not be disposed of via wastewater or household waste. Dispose of the container and any unused medicine in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the container and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of SeptaNasal
- The active substances are xylometazoline hydrochloride and dexpanthenol.
Each ml of nasal spray solution contains 1 mg of xylometazoline hydrochloride and 50 mg of dexpanthenol.
One spray contains 0.1 ml of nasal spray solution with 0.1 mg of xylometazoline hydrochloride and 5.0 mg of dexpanthenol.
- The other ingredients are potassium dihydrogen phosphate, disodium phosphate dodecahydrate, and water for injection.
Appearance of the product and contents of the pack
The nasal spray solution is a clear, colorless liquid.
SeptaNasal is available in 10 ml nasal spray solution packs, in a plastic spray container with a pump spray.
10 ml of nasal spray solution are sufficient for 90 sprays.
Marketing authorisation holder and manufacturer
Krka, d.d., Novo mesto
Šmarješka cesta 6
8501 Novo mesto
Slovenia
You can obtain further information on this medicine by contacting the local representative of the marketing authorisation holder:
KRKA Farmacéutica, S.L., Calle de Anabel Segura 10, 28108 Alcobendas, Madrid, Spain
This medicine is authorised in the Member States of the European Economic Area under the following names:
Member State | Medicine name |
Spain | SeptaNasal 1 mg/ml + 50 mg/ml nasal spray solution |
Date of last revision of this leaflet:November 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SEPTANASAL 1 mg/ml + 50 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 0.5 mg/ml + 0.6 mg/mlActive substance: xylometazolineManufacturer: Haleon Spain S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.5+0.5 mg/ml+mg/mlActive substance: oxymetazolineManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription required
Online doctors for SEPTANASAL 1 mg/ml + 50 mg/ml NASAL SPRAY SOLUTION
Discuss questions about SEPTANASAL 1 mg/ml + 50 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











