
Xilometazoline + Dexpanthenol Teva
Ask a doctor about a prescription for Xilometazoline + Dexpanthenol Teva

How to use Xilometazoline + Dexpanthenol Teva
Leaflet accompanying the packaging: patient information
Xylometazoline + Dexpanthenol Teva, (1 mg + 50 mg)/mL,
nasal spray, solution
Xylometazoline hydrochloride+ Dexpanthenol
For use in adults and children over 6 years of age.
Please read carefully the contents of the leaflet before using the medicine, as it contains
important information for the patient.
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor, pharmacist, or nurse.
- You should keep this leaflet, so you can read it again if you need to.
- If you need advice or more information, you should speak to your pharmacist.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
- If after 7 days there is no improvement or you feel worse, you should contact your doctor.
Table of contents of the leaflet
- 1. What is Xylometazoline + Dexpanthenol Teva and what is it used for
- 2. Important information before using Xylometazoline + Dexpanthenol Teva
- 3. How to use Xylometazoline + Dexpanthenol Teva
- 4. Possible side effects
- 5. How to store Xylometazoline + Dexpanthenol Teva
- 6. Contents of the packaging and other information
1. What is Xylometazoline + Dexpanthenol Teva and what is it used for
Xylometazoline + Dexpanthenol Teva contains two active substances: xylometazoline hydrochloride and dexpanthenol. Xylometazoline constricts blood vessels, which leads to a reduction in nasal mucosal swelling. The active substance dexpanthenol is a derivative of pantothenic acid, a vitamin that supports wound healing and has a protective effect on mucous membranes.
Xylometazoline + Dexpanthenol Teva is used to reduce nasal mucosal swelling in rhinitis and as an adjunctive treatment in wound healing of the skin and mucous membranes, as well as in the treatment of nasal congestion (vasomotor rhinitis) and in the treatment of impaired breathing through the nose after nasal surgery.
Xylometazoline + Dexpanthenol Teva is indicated for use in adults and children over 6 years of age.
If after 7 days there is no improvement or you feel worse, you should contact your doctor.
2. Important information before using Xylometazoline + Dexpanthenol Teva
When not to use Xylometazoline + Dexpanthenol Teva:
- if you are allergic to xylometazoline hydrochloride, dexpanthenol, or any of the other ingredients of this medicine (listed in section 6),
- if you have atrophic rhinitis (a chronic form of rhinitis that causes dryness of the nasal mucosa with crust formation),
- if you have recently undergone neurosurgical procedures (transsphenoidal hypophysectomy or other surgery that exposes the dura mater),
- in children under 6 years of age.
Warnings and precautions
Before starting to use Xylometazoline + Dexpanthenol Teva, you should discuss it with your doctor or pharmacist if you are:
- taking certain medicines for depression, known as monoamine oxidase inhibitors (MAOIs) or other medicines that may increase blood pressure (e.g., doksapram, ergotamine, oxytocin).
- taking medicines that lower blood pressure (e.g., methyldopa).
- have increased intraocular pressure, especially if you have narrow-angle glaucoma,
- have severe cardiovascular diseases (e.g., long QT syndrome) or high blood pressure (hypertension),
- have a pheochromocytoma (a tumor of the adrenal gland),
- have metabolic disorders, such as hyperthyroidism and diabetes,
- have a metabolic disorder called porphyria (a disorder that affects the skin and/or nervous system),
- have prostatic hyperplasia (enlargement of the prostate gland).
The use of this medicine in the case of chronic rhinitis should be carried out under medical supervision due to the risk of damage to the nasal mucosa (tissue inside the nose).
Children
Do not use in children under 6 years of age. For children between 2 and 6 years of age, a suitable nasal spray with a lower concentration of xylometazoline hydrochloride is available.
You should avoid prolonged use or use in higher doses than recommended, especially in children. Higher doses should only be given after consulting a doctor.
Xylometazoline + Dexpanthenol Teva and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Concomitant use of Xylometazoline + Dexpanthenol Teva with certain medicines, e.g., mood-enhancing medicines (monoamine oxidase inhibitors of the tranylcypromine type or tricyclic antidepressants), as well as medicines that may increase blood pressure (e.g., doksapram, ergotamine, oxytocin), may cause an increase in blood pressure as a result of the effect on the cardiovascular system.
Do not use this medicine concomitantly with medicines that lower blood pressure (e.g., methyldopa), due to the possible effect of xylometazoline on blood vessels (increased blood pressure).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, you should ask your doctor or pharmacist for advice before using this medicine.
Due to the lack of data on the safety of use, this medicine should not be used in pregnant women.
It is not known whether xylometazoline hydrochloride passes into breast milk, so Xylometazoline + Dexpanthenol Teva should not be used during breastfeeding.
Driving and using machines
If Xylometazoline + Dexpanthenol Teva is used as directed, it is not expected to have a negative effect on the ability to drive or use machines.
3. How to use Xylometazoline + Dexpanthenol Teva
This medicine should always be used exactly as described in this patient leaflet or as directed by your doctor or pharmacist. If you are unsure, you should ask your doctor or pharmacist.
Recommended dose If your doctor has not prescribed otherwise, adults and children over 6 years of age should use one spray of
Xylometazoline + Dexpanthenol Teva into each nostril, as needed, butno more than three times a day.The dosage depends on the individual sensitivity and response of the patient to the medicine.
Caution
Before the first use or if the spray has not been used for more than 7 days, you should press the pump several times until a uniform mist appears. For subsequent applications, the spray with the measured dose is ready for immediate use.
Before using the nasal spray, gently blow your nose. It is recommended to take the last dose of each day before going to bed.
For hygiene reasons and to avoid infection, each bottle with an applicator should be used by only one person.
Duration of use
Do not use Xylometazoline + Dexpanthenol Teva for more than 7 days, unless your doctor has explicitly advised you to continue using it.
Re-use should only occur after a few days' break.
When using in children, you should always consult a doctor regarding the duration of use.
Continuous use of Xylometazoline + Dexpanthenol Teva may lead to chronic swelling and ultimately cause atrophy of the nasal mucosa.
Patients with increased intraocular pressure (with glaucoma, especially with narrow-angle glaucoma) should consult a doctor before using this medicine.
If the effect of Xylometazoline + Dexpanthenol Teva is too strong or too weak, you should consult a doctor or pharmacist.
Use of a higher dose of Xylometazoline + Dexpanthenol Teva than recommended
In the event of using a higher dose of Xylometazoline + Dexpanthenol Teva than recommended or accidental oral ingestion, the following symptoms may occur: constriction of the pupils, dilation of the pupils, fever, sweating, pallor, cyanosis of the lips, nausea, convulsions, cardiovascular disorders, e.g., arrhythmias (tachycardia, bradycardia, cardiac arrhythmia), circulatory collapse, cardiac arrest, high blood pressure (hypertension); respiratory disorders (pulmonary edema, respiratory disorders), psychiatric disorders. Additionally, drowsiness, decreased body temperature, slowed heart rate, a drop in blood pressure similar to shock, respiratory arrest, and loss of consciousness (coma) may occur.
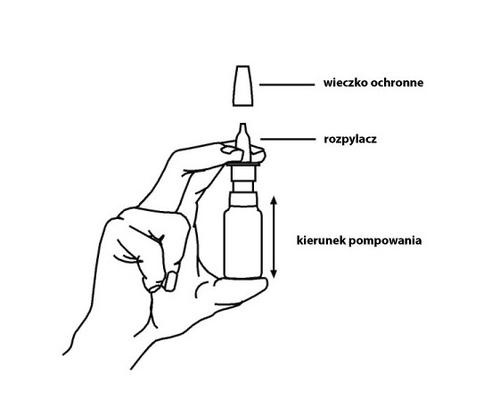 | Method of administration The medicine is intended for nasal use. After removing the protective cap, the spray nozzle (applicator) should be placed in the nostril and the pump should be pressed once. During spraying, you should breathe gently through your nose. After each use, the dosing tip should be carefully wiped with a clean cloth and the protective cap should be put back on. |
If you suspect that you have used a higher dose of the medicine than recommended, you should contact your doctor immediately. If necessary, the doctor will start appropriate treatment.
Missing a dose of Xylometazoline + Dexpanthenol Teva
You should not use a double dose to make up for a missed dose. You should continue treatment as described in the instructions for use.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of side effects is defined as follows:
Very common: may affect more than 1 in 10 people,
Common: may affect up to 1 in 10 people,
Uncommon: may affect up to 1 in 100 people,
Rare: may affect up to 1 in 1,000 people,
Very rare: may affect up to 1 in 10,000 people,
Frequency not known: frequency cannot be estimated from the available data.
Immune system disorders:
Uncommon: allergic reactions (hypersensitivity) - skin and mucous membrane swelling, skin rash, itching
Psychiatric disorders:
Very rare: excitement, insomnia, hallucinations (especially in children)
Nervous system disorders:
Very rare: fatigue (drowsiness, sedation), headache, convulsions (especially in children)
Cardiac disorders:
Rare: palpitations, accelerated heart rate (tachycardia)
Very rare: arrhythmias (cardiac arrhythmia)
Vascular disorders:
Rare: increased blood pressure (hypertension)
Respiratory, thoracic, and mediastinal disorders:
Very rare: increased swelling of the nasal mucosa after the effect of the medicine has stopped, nosebleeds
Frequency not known: burning and dryness of the nasal mucosa, sneezing
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301, Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Xylometazoline + Dexpanthenol Teva
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label after: "Expiry date" or "EXP". The expiry date refers to the last day of the month.
There are no special precautions for storage.
Shelf life after first opening: 6 months.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Xylometazoline + Dexpanthenol Teva contains
The active substances are: xylometazoline hydrochloride + dexpanthenol.
One spray of the nasal spray (0.1 mL solution) contains 0.1 mg xylometazoline hydrochloride and 5.0 mg dexpanthenol. 1 mL solution contains: xylometazoline hydrochloride 1 mg and dexpanthenol 50 mg.
The other ingredients of the medicine are:
- Potassium dihydrogen phosphate
- Disodium hydrogen phosphate heptahydrate
- Water for injections
What Xylometazoline + Dexpanthenol Teva looks like and contents of the pack
One bottle with 10 mL solution contains at least 90 single sprays.
Xylometazoline + Dexpanthenol Teva is a clear, almost colorless solution in a 10 mL round plastic bottle, containing 10 mL solution, sealed with a spray pump (applicator) with a protective cap.
Marketing authorization holder:
Teva B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
Manufacturer:
Merckle GmbH
Ludwig-Merckle-Strasse 3
89143 Blaubeuren
Germany
PLIVA Hrvatska d.o.o.
(PLIVA Croatia Ltd.)
Prilaz baruna Filipovica 25
10000 Zagreb
Croatia
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany: Xylometazolin/Dexpanthenol Teva 1 mg/mL + 50 mg/mL Nasenspray Lösung
Poland: Xylometazoline + Dexpanthenol Teva
To obtain more detailed information on the medicine and its names in other Member States of the European Economic Area, you should contact the representative of the marketing authorization holder:
Teva Pharmaceuticals Polska Sp. z o.o.,
ul. Emilii Plater 53,
00-113 Warsaw,
phone (22) 345 93 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterMerckle GmbH PLIVA Hrvatska d.o.o. (PLIVA Croatia Ltd.)
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Xilometazoline + Dexpanthenol TevaDosage form: Aerosol, (0.05 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.1 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.5 mg + 0.6 mg)/mlActive substance: xylometazolinePrescription not required
Alternatives to Xilometazoline + Dexpanthenol Teva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Xilometazoline + Dexpanthenol Teva in Ukraine
Alternative to Xilometazoline + Dexpanthenol Teva in Spain
Online doctors for Xilometazoline + Dexpanthenol Teva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Xilometazoline + Dexpanthenol Teva – subject to medical assessment and local rules.








