
RHINOVIN DUO 0.5 mg/ml + 0.6 mg/ml NASAL SPRAY SOLUTION

How to use RHINOVIN DUO 0.5 mg/ml + 0.6 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Rhinovin Duo 0.5 mg/ml + 0.6 mg/ml Nasal Spray Solution
Xylometazoline Hydrochloride / Ipratropium Bromide
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 7 days of treatment.
Contents of the Package Leaflet
- What is Rhinovin Duo and what is it used for
- What you need to know before you start using Rhinovin Duo
- How to use Rhinovin Duo
- Possible side effects
- Storage of Rhinovin Duo
- Contents of the pack and further information
1. What is Rhinovin Duo and what is it used for
Rhinovin Duo is an association of 2 active substances: ipratropium that reduces nasal secretion and xylometazoline that has a decongestant effect.
Rhinovin Duo is used for the treatment of congestion and runny nose (rhinorrhea) related to the common cold.
2. What you need to know before you start using Rhinovin Duo
Do not use Rhinovin Duo:
- In children under 18 years, as there is no adequate information available
- If you are allergic to xylometazoline hydrochloride or ipratropium bromide or to any of the other components of this medicine (listed in section 6).
- If you are allergic to atropine or similar substances, e.g. hyoscine and scopolamine.
- If you have had your pituitary gland removed through surgical operation through the nose.
- If you have undergone brain surgery through the nose or mouth.
- If you have glaucoma (high intraocular pressure).
- If you suffer from severe nasal dryness (inflammatory nasal dryness, dry rhinitis or atrophic rhinitis).
Warnings and precautions
Rhinovin Duo may cause sleep disturbances, dizziness, tremors, irregular heartbeat or increased blood pressure in case of sensitivity to nasal decongestant medications. Consult your doctor if these symptoms occur and are bothersome.
Consult your doctor or pharmacist before using Rhinovin Duo if you have:
- Any cardiovascular disease (e.g. long QT syndrome)
- High blood pressure
- Diabetes
- Hyperthyroidism (elevated thyroid hormone release).
- Difficulty urinating and/or prostate hypertrophy
- Narrow-angle glaucoma.
- Predisposition to nasal bleeding
- Intestinal obstruction (paralytic ileus).
- Cystic fibrosis.
- Benign tumor of the adrenal gland that produces large amounts of adrenaline and noradrenaline (pheochromocytoma) or exceptional sensitivity to adrenaline and noradrenaline
A hypersensitivity reaction may occur, which manifests as a red, itchy rash, with elevated skin inflammation (urticaria), difficulty breathing or speaking, difficulty swallowing due to inflammation of the lips, face or throat. These symptoms may appear individually or combined in the form of a severe allergic reaction. If this happens, discontinue treatment with Rhinovin Duo immediately (see section 4).
Rhinovin Duo should not be used for more than 7 consecutive days. If symptoms persist, consult your doctor. Prolonged use or excessive dosing may cause rebound nasal congestion or worsening of this symptom, and inflammation of the nasal mucosa.
Avoid spraying Rhinovin Duo around the eyes. If this happens, rinse your eyes with cold water. You may experience temporary blurred vision, irritation, pain, and redness in the eyes. If this occurs, contact your doctor for advice. Narrow-angle glaucoma may also worsen.
Children and adolescents
The use of Rhinovin Duo is not recommended in children and adolescents under 18 years, as there is no adequate information available on safety and efficacy.
Using Rhinovin Duo with other medicines
Tell your doctor or pharmacist if you are using or have recently used or may need to use any other medicine. Rhinovin Duo may influence or be influenced by:
- Monoamine oxidase inhibitors, used to treat depression. If you are taking or have taken this type of medicine in the last two weeks, a dangerous increase in blood pressure may occur.
- Tricyclic and tetracyclic antidepressants (if you are taking or have taken this type of medicine in the last two weeks, dangerous increases in blood pressure may occur).
- Medicines used to reduce dizziness in transportation (medicines containing anticholinergic substances).
- Medicines used to treat intestinal disorders (particularly those used in the treatment of altered intestinal motility) (medicines containing anticholinergic substances).
- Medicines used to treat respiratory disorders (beta-2 agonists) such as asthma or chronic obstructive pulmonary disease (COPD), as they may worsen glaucoma if you have a history of narrow-angle glaucoma.
If you are using any of the above-mentioned medicines, consult your doctor before using Rhinovin Duo.
Pregnancy and breastfeeding
Rhinovin Duo should not be used during pregnancy unless your doctor has prescribed it. During the breastfeeding period, do not use Rhinovin Duo unless your doctor decides that the benefits are greater than the possible risks to the child.
If you are pregnant or breastfeeding, think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
With the administration of Rhinovin Duo, visual disturbances (including blurred vision and mydriasis), dizziness, and fatigue have been reported. Patients should be warned not to drive, use machines, or engage in certain activities if they are affected by these symptoms, as they may put themselves and others at risk.
3. How to use Rhinovin Duo
Follow exactly the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
Adults: One spray in each nostril as needed, up to 3 sprays per day, for a maximum of 7 days. At least 6 hours should pass between each dose. Do not exceed more than 3 applications per day in each nostril.
Do not exceed the established dose, use the lowest dose you need to treat your symptoms, and use it for the shortest time necessary to achieve the desired effect.
Treatment duration:
Do not use this medicine for more than 7 days.
It is recommended to stop treatment with Rhinovin Duo as soon as symptoms improve, even before the maximum treatment duration of 7 days, in order to minimize the risk of adverse reactions.
If your symptoms worsen or do not improve after 7 days, consult your doctor.
If you think the effect of Rhinovin Duo is too strong or too weak, consult your doctor or pharmacist.
Instructions for use:
Vertically operated pump with two fingers:
- Remove the protector
- Do not cut the end of the valve. The dosing valve is prepared to be operated before use
Before the first application, load the pump by operating it 4 times. Once loaded, the pump remains loaded through normal daily use during the treatment period. If the spray does not come out during operation, or if the medicine has not been used for more than 6 days, the pump should be reloaded by operating it 4 times, just like before the first application.
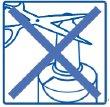
- Blow your nose.
- Hold the bottle upright with your thumb under the base and the nozzle between your two fingers.
- Move your head forward slightly and insert the nozzle into one of the nostrils.
- Press the sprayer while gently inhaling the product through your nose.
- Repeat this procedure (steps 1 to 4) in the other nostril.
- Clean and dry the nozzle before putting the cap back on after use.
Laterally operated pump with the thumb:
Remove the cap.
Before using for the first time
Load the pump by operating it 5 times. Once loaded, the pump will remain loaded normally throughout the daily treatment period.
- Blow your nose.
- Hold the bottle vertically with your thumb on the operating button.
- To avoid dripping, stand upright and insert the spray nozzle into a nostril.
- Press the button to spray and inhale gently through your nose at the same time.
Repeat this process (steps 2 to 4) in the other nostril.
- After each use, clean and dry the nozzle.
- Put the protective cap back on until you hear a "click".
If the spray does not come out during operation, or if the medicine has not been used for more than 7 days, it will be necessary to reload the pump with 2 operations.
If the full spray is not administered, the dose should not be repeated.
To avoid possible spread of infection, the spray should only be used by one person. Avoid spraying Rhinovin Duo around the eyes.
The effect appears within 5-15 minutes.
If you use more Rhinovin Duo than you should
If you or someone around you takes more medicine than they should, contact your doctor, hospital, or emergency service to assess the risk. It is recommended that you take the package leaflet, packaging, or carton with you. This is especially important in the case of children, as they are more susceptible to developing adverse effects than adults.
The symptoms of overdose are: severe dizziness, sweating, sudden drop in body temperature, headache, slow heartbeat, rapid heartbeat, difficulty breathing, coma, convulsions, hypertension (high blood pressure) that may be followed by hypotension (low blood pressure).
Other symptoms may include dry mouth, eye focusing problems, and hallucinations.
In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service (telephone: 91.5620420), indicating the medicine and the amount ingested.
If you forget to take Rhinovin Duo
Do not apply a double dose to make up for a missed application.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop treatment with Rhinovin Duo and consult your doctor immediately if you experience any of the following side effects:
- Palpitations or increased heart rate (affect less than 1 in 100 patients)
- Signs of allergic reaction such as difficulty breathing, speaking, or swallowing; inflammation of the face, lips, tongue, or throat; severe skin itching, with red rash or hives (frequency cannot be determined from available data).
- Visual disturbances (including blurred vision, worsening of glaucoma, or increased ocular pressure), halos/circles with rainbow colors around bright lights, and/or eye pain (frequency cannot be determined from available data).
The most common side effects are nasal bleeding and nasal dryness. Many of the reported side effects are also symptoms of the common cold.
Very common (affect more than 1 in 10 patients):
- Nasal bleeding
Common (affect 1 in 10 patients):
- Nasal discomfort, nasal congestion, dry nose, nasal pain.
- Dry mouth, dry throat or irritation.
- Altered taste and headache, dizziness, local burning sensation.
- Nausea
Uncommon (affect between 1 in 100 patients):
- Nasal ulcer, sneezing, throat pain, cough, hoarseness, stomach upset, nausea
- Altered olfactory sensation, agitation
- Discomfort, fatigue
- Insomnia
- Eye irritation, dry eyes, eye swelling, eye redness
- Palpitations, increased heart rate
Rare (affect 1 in 1,000 patients):
- Nasal discharge
Very rare (affect less than 1 in 10,000 patients):
- Drug reaction such as swelling, rash, and itching
- Visual impairment
Frequency not known (cannot be calculated from available data):
- Urticaria
- Discomfort around the nose
- Difficulty swallowing
- Chest discomfort, thirst
- Sudden muscle spasm in the throat, throat swelling
- Irregular heartbeat
- Eye focusing problems, pupil dilation, flashing lights, increased ocular pressure, glaucoma, blurred vision, halos around bright lights, and eye pain.
- Difficulty urinating
In order to minimize the risk of adverse reactions, it is recommended to discontinue treatment with Rhinovin Duo when symptoms improve
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rhinovin Duo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and label after "Expiration Date". The expiration date is the last day of the month indicated. This date applies even if the package has been opened.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Content and Additional Information
Rhinovin Duo Composition
The active ingredients are xylometazoline hydrochloride and ipratropium bromide.
1 ml contains 0.5 mg of xylometazoline hydrochloride and 0.6 mg of ipratropium bromide.
1 spray contains 70 micrograms of xylometazoline hydrochloride and 84 micrograms of ipratropium bromide.
The other components (excipients) are: disodium edetate, glycerol (85%), purified water, sodium hydroxide, and hydrochloric acid.
Product Appearance and Container Content
Rhinovin Duo is a clear solution.
The bottle contains approximately 70 sprays.
Rhinovin Duo is available as a 10 ml nasal spray with a vertically operated pump with two fingers and as a 10 ml nasal spray with a laterally operated pump with the thumb (with protective cap).
Marketing Authorization Holder and Manufacturer
Haleon Spain, S.A., Paseo de la Castellana, 259D, 32nd floor - 28046 – Madrid – Spain.
Manufacturer
Haleon Denmark ApS
Delta Park 37, 2665 Vallensbæk Strand - Denmark
Or
Haleon Belgium NV
Da Vincilaan 5,
1930 Zaventem, Belgium
Or
Haleon - Gebro Consumer Health GmbH
Bahnhofbichl, 13
6391 Fieberbrunn
Austria
Or
Haleon Germany GmbH
Barthstraße 4 – 80339 Munich - Germany
This medication is authorized in EEE member states under the following names:
Austria: Otrivin Duo 0.5 mg/ml + 0.6 mg/ml Nasenspray, Lösung
Belgium: Otrivine Duo 0.5mg/ml + 0.6mg/ml solution pour pulvérisation nasale
Cyprus: Otrivin Advance (0.5mg/ml + 0.6mg/ml) nasal spray solution
Czech Republic: Otrivin Rhinostop
Denmark: Otrivin Comp naesespray, oplosning
Estonia: Otrivin Total
Greece: Otrivin Advance 0.5mg/ml + 0.6mg/ml Ρινικ? εκν?φωμα, δι?λυμα
Spain: Rhinovín Duo 0.5 mg/ml + 0.6 mg/ml solución para pulverización nasal
Finland: Otrivin Comp 0.5mg/ml + 0.6mg/ml nenäsumute, liuos
Hungary: Otrivin Komplex 0.5mg/ml + 0.6mg/ml oldatos orrspray
Ireland: Otrivine Extra Dual Relief 0.5 mg/ml, 0.6 mg/ml Nasal Spray
Iceland: Otrivin Comp 0.5mg/ml + 0.6mg/ml nefúði lausn
Italy: Rinazina Doppia Azione 0.5mg/ml + 0.6mg/ml spray nasale, soluzione
Lithuania: OtriDuo 0.5 mg/0.6 mg/ml nosies purškalas, tirpalas
Luxembourg: Otrivine Duo 0.5mg/ml + 0.6mg/ml solution pour pulvérisation nasale
Latvia: Otrivin Total 0.5 mg/ml + 0.6mg/ml deguna pilieni skidums
Malta: Otrivine Extra dual Relief 0.5 mg/ml, 0.6 mg/ml Nasal Spray
Netherlands: Otrivin Duo Xylometazoline hydrochloride & Ipratropium bromide, 0.5/0.6 mg/ml, neusspray, oplossing
Norway: Otrivin Comp 0.5mg/ml + 0.6mg/ml nesespray, oppløsning
Poland: Otrivin Ipra MAX
Portugal: Vibrocil ActilongDuo 0.5mg/ml + 0.6mg/ml solução para pulverização
Romania: Vibrocil Duo 0.5mg/ml + 0.6mg/ml spray nazal, solutie
Sweden: Otrivin Comp 0.5mg/ml + 0.6 mg/ml nässpray lösning
Slovenia: Otrivin Duo 0.5 mg/0.6 mg v 1 ml pršilo za nos, raztopina
Slovakia: Otrivin Complete
United Kingdom (Northern Ireland): Otrivine Extra Dual Relief 0.5 mg/ml, 0.6 mg/ml Nasal Spray
Date of the Last Revision of this Leaflet:June 2023
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RHINOVIN DUO 0.5 mg/ml + 0.6 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 1 mg/ml + 50 mg/mlActive substance: xylometazolineManufacturer: Krka D.D. Novo MestoPrescription not requiredDosage form: NASAL PRODUCT, 0.5+0.5 mg/ml+mg/mlActive substance: oxymetazolineManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription required
Online doctors for RHINOVIN DUO 0.5 mg/ml + 0.6 mg/ml NASAL SPRAY SOLUTION
Discuss questions about RHINOVIN DUO 0.5 mg/ml + 0.6 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











