
SEFFALAIR SPIROMAX 12.75 micrograms/202 micrograms INHALATION POWDER

How to use SEFFALAIR SPIROMAX 12.75 micrograms/202 micrograms INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Seffalair Spiromax 12.75micrograms/202micrograms inhalation powder
salmeterol/fluticasone propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Seffalair Spiromax is and what it is used for
- What you need to know before you use Seffalair Spiromax
- How to use Seffalair Spiromax
- Possible side effects
- Storing Seffalair Spiromax
- Contents of the pack and other information
1. What Seffalair Spiromax is and what it is used for
Seffalair Spiromax contains 2 active substances: salmeterol and fluticasone propionate:
- Salmeterol is a long-acting bronchodilator. Bronchodilators help to keep the airways open, making it easier to breathe. The effects of salmeterol last for at least 12 hours.
- Fluticasone propionate is a corticosteroid that reduces swelling and irritation in the lungs.
Seffalair Spiromax is used to treat asthma in adults and adolescents aged 12 years and older.
Seffalair Spiromax helps to prevent shortness of breath and wheezing.Do not use it to relieve an asthma attack. If you have an asthma attack, use a fast-acting relief inhaler (such as salbutamol), which you should always carry with you.
2. What you need to know before you use Seffalair Spiromax
Do not use Seffalair Spiromax
- if you are allergic to salmeterol, fluticasone propionate, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before using Seffalair Spiromax if you have:
- A heart condition, including a fast or irregular heartbeat
- An overactive thyroid gland
- High blood pressure
- Diabetes (Seffalair Spiromax may increase blood sugar levels)
- Low potassium levels in the blood
- Tuberculosis (TB), currently or in the past, or other lung infections
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Do not give Seffalair Spiromax to children or adolescents under 12 years of age, as it has not been studied in this age group.
Other medicines and Seffalair Spiromax
Tell your doctor, nurse, or pharmacist if you are taking, have recently taken, or might take any other medicines. It may not be suitable to use Seffalair Spiromax with other medicines.
Tell your doctor if you are taking the following medicines before starting to use Seffalair Spiromax:
- Beta-blockers (such as atenolol, propranolol, and sotalol). Beta-blockers are mainly used for high blood pressure or heart problems such as angina.
- Medicines to treat infections (such as ritonavir, ketoconazole, itraconazole, and erythromycin). Some of these medicines may increase the amount of salmeterol or fluticasone propionate in your body. This may increase the side effects of Seffalair Spiromax, including irregular heartbeats, or worsen its side effects.
- Corticosteroids (by mouth or by injection). Recent use of these medicines may increase the risk that Seffalair Spiromax will affect the adrenal glands, reducing the amount of steroid hormones they produce (adrenal suppression).
- Diuretics, medicines that increase urine production and are used to treat high blood pressure.
- Other bronchodilators (such as salbutamol).
- Xanthine medicines such as aminophylline and theophylline, often used to treat asthma.
Some medicines may increase the effects of Seffalair Spiromax, so your doctor may want to keep a close eye on you if you are taking these medicines (including some medicines for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor, nurse, or pharmacist for advice before using this medicine.
It is not known whether this medicine passes into breast milk. If you are breastfeeding, ask your doctor, nurse, or pharmacist for advice before using this medicine.
Driving and using machines
Seffalair Spiromax is unlikely to affect your ability to drive or use machines.
Seffalair Spiromax contains lactose
Each dose of this medicine contains approximately 5.4 milligrams of lactose. If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
3. How to use Seffalair Spiromax
Follow the instructions for using this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist again.
The recommended dose is one inhalation twice a day.
- Seffalair Spiromax is for regular long-term use. Use it every day to keep your asthma under control. Do not take more than the recommended dose. If you are unsure, ask your doctor, nurse, or pharmacist again.
- Do not stop using Seffalair Spiromax or reduce your dose without talking to your doctor or nurse first.
- Seffalair Spiromax should be inhaled through the mouth.
Your doctor or nurse will help you manage your asthma. The doctor or nurse will change the medicine you take with the inhaler if you need a different dose to control your asthma properly. However, do not change the number of inhalations prescribed by your doctor or nurse without talking to them first.
If your asthma gets worse or you breathe more difficulty, tell your doctor immediately.If you notice more wheezing, feel tightness in the chest more often, or need to use your fast-acting relief medicine more often, it may be a sign that your asthma is getting worse, and you may become seriously ill. Continue using Seffalair Spiromax, but do not increase the number of inhalations you take. See your doctor immediately, as you may need additional treatment.
Instructions for use
Training
Your doctor, nurse, or pharmacist should train you in the use of the inhaler, including how to inhale a dose effectively. This training is important to ensure that you receive the dose you need. If you have not received this training, talk to your doctor, nurse, or pharmacist to teach you how to use the inhaler properly before using it for the first time.
Your doctor, nurse, or pharmacist should also check from time to time that you are using the Spiromax device correctly and as prescribed. If you are not using Seffalair Spiromax correctly or not inhaling through sufficiently forcedinspirations, you may not be getting enough medicine into your lungs. This means that the medicine will not relieve your asthma as it should.
Preparing Seffalair Spiromax
Before using Seffalair Spiromax for the first time, you need to prepare it for use as follows:
- Check the dose indicator to make sure the inhaler contains 60 inhalations.
- Write the date you opened the foil pouch on the label attached to the inhaler.
- You do not need to shake the inhaler before use.
How to take an inhalation
- Hold the inhalerwith the semi-transparent yellow mouthpiece cover at the bottom.
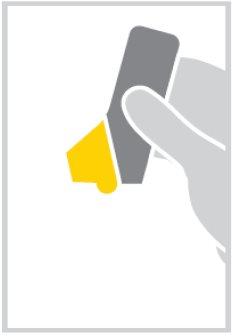
- Open the mouthpiece cover by folding it down until you hear a click. This will load a dose of medicine. The inhaler is now ready to use.
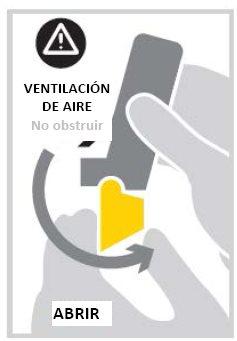
- Breathe out gently (as much as you can). Do not breathe out through the inhaler.
- Put the mouthpiece in your mouth and close your lips around it. Be careful not to block the air vents.
Breathe in as deeply and forcefully as you can.
It is important that you take a forcedinspiration.
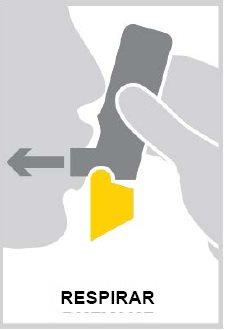
- Take the inhaler out of your mouth. You may notice a taste when you take the inhalation.
- Hold your breath for 10 seconds or as long as you comfortably can.
- Breathe out gently(do not breathe out through the inhaler).
- Close the mouthpiece cover.
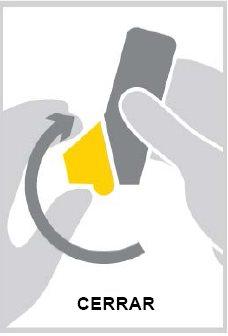
- After each dose, rinse your mouth with water and spit it out or brush your teeth before rinsing.
- Do not try to take the inhaler apart or remove or twist the mouthpiece cover.
- The cover is fixed to the inhaler and should not be removed.
- Do not use the Spiromax device if it has been damaged or if the mouthpiece has been taken apart.
- Do not open and close the mouthpiece cover unless you are about to use the inhaler.
Cleaning the Spiromax device
Keep the inhaler clean and dry.
If necessary, you can clean the mouthpiece of the inhaler with a dry cloth or paper tissue after use.
When to start using a new Seffalair Spiromax
- The dose indicator on the back of the device shows how many doses (inhalations) are left in the inhaler, starting from 60 inhalations when it is full and ending with 0 (zero) when it is empty.

- The dose indicator shows the number of inhalations left only in even numbers. The spaces between the even numbers represent the odd number of inhalations left.
- When there are 20 or fewer inhalations left, the numbers will appear in red on a white background. When the red numbers appear in the window, go to your doctor or nurse to get a new inhaler.
Note:
- The mouthpiece makes a click even when the inhaler is empty.
- If you open and close the mouthpiece without taking an inhalation, the dose indicator will also count this as a dose. This dose will be trapped inside the inhaler for when you take the next inhalation. It is not possible to accidentally take too much medicine or a double dose in one inhalation.
If you use more Seffalair Spiromax than you should
It is important that you take the dose that your doctor or nurse has prescribed. Do not exceed the prescribed dose without talking to a doctor. If you accidentally take more doses than recommended, talk to your nurse, doctor, or pharmacist. You may notice that your heart beats faster than normal and that you feel shaky. You may also feel dizzy, have a headache, feel weak, or have joint pain.
If you have repeatedly taken too many doses of Seffalair Spiromax over a long period, talk to your doctor or pharmacist, as taking too much Seffalair Spiromax can reduce the amount of steroid hormones produced by the adrenal glands.
If you forget to use Seffalair Spiromax
If you forget to take a dose, take it as soon as you remember, but do nottake a double dose to make up for forgotten doses. If it is almost time for your next dose, just take the next dose at the usual time.
If you stop using Seffalair Spiromax
It is very important that you take Seffalair Spiromax every day as directed. Continue to take it until your doctor tells you to stop. Do not stop using Seffalair Spiromax or suddenly reduce the dose.This could make your breathing worse.
Additionally, if you suddenly stop using Seffalair Spiromax or reduce your dose, you may experience (in very rare cases) problems due to a decrease in the amount of steroid hormones produced in the adrenal glands (adrenal insufficiency), which can sometimes cause side effects.
These side effects may include:
- Stomach pain
- Tiredness and loss of appetite, nausea
- Vomiting and diarrhea
- Weight loss
- Headache or drowsiness
- Low blood sugar levels
- Low blood pressure and seizures (epileptic fits)
In stressful situations for your body, such as fever, accident, or injury, infection, or surgery, adrenal insufficiency may worsen, and you may also experience the previously mentioned side effects.
If you experience any side effects, talk to your doctor or pharmacist. To avoid these symptoms, your doctor may prescribe more corticosteroids in tablet form (such as prednisolone).
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. To reduce the likelihood of adverse effects, your doctor will prescribe the lowest dose of this combination of drugs to control asthma.
Allergic Reactions: you may notice that you suddenly breathe worse immediately after using Seffalair Spiromax.You may have a lot of wheezing and coughing or feel short of breath. You may also experience itching, a skin rash (hives), and swelling (usually of the face, lips, tongue, or throat), or notice that your heart beats very fast or feel faint or dizzy (which can lead to syncope or loss of consciousness). If you experience any of these effects or they appear suddenly after using Seffalair Spiromax, stop treatment with Seffalair Spiromax and inform your doctor immediately.Allergic reactions to Seffalair Spiromax are rare (they can affect up to 1 in 100 people).
The following are other adverse effects:
Frequent(can affect up to 1 in 10 people)
- A fungal infection (candidiasis) that causes painful, raised patches in the mouth and throat, as well as pain in the tongue and hoarseness and throat irritation. Rinsing your mouth with water and spitting it out immediately or brushing your teeth after each inhalation may be helpful. Your doctor may prescribe an antifungal medication to treat candidiasis.
- Muscle pain.
- Back pain.
- Flu.
- Low potassium levels in the blood (hypokalemia).
- Nasal inflammation (rhinitis).
- Inflammation of the paranasal sinuses (sinusitis).
- Inflammation of the nose and throat (nasopharyngitis).
- Headache.
- Cough.
- Throat irritation.
- Pain or inflammation in the back of the throat.
- Hoarseness or loss of voice.
- Dizziness.
Uncommon(can affect up to 1 in 100 people)
- Increased sugar (glucose) in the blood (hyperglycemia). If you have diabetes, you may need to monitor your blood sugar more frequently and possibly adjust your usual diabetes treatment.
- Cataracts (clouding of the eye lens).
- Very fast heartbeat (tachycardia).
- Feeling of trembling and rapid heartbeat (palpitations): these are usually harmless and decrease as treatment continues.
- Feelings of worry or anxiety.
- Behavioral changes, such as unusual activity and irritability (although these effects occur mainly in children).
- Sleep disturbances.
- Allergic rhinitis.
- Nasal congestion (stuffy nose).
- Irregular heartbeat (atrial fibrillation).
- Chest infection.
- Pain in the limbs (arms or legs).
- Stomach pain.
- Indigestion.
- Skin damage and peeling.
- Skin inflammation.
- Throat inflammation, usually characterized by sore throat (pharyngitis).
Rare(can affect up to 1 in 1,000 people)
- Difficulty breathing or worsening wheezing immediately after using Seffalair Spiromax.If this occurs, stop treatment with the Seffalair Spiromax inhaler.Use your rapid-acting "rescue" inhaler to help you breathe and inform your doctor immediately.
- Seffalair Spiromax may affect the normal production of steroid hormones in the body, especially if you have taken high doses for prolonged periods. Among the effects are:
- Slowed growth in children and adolescents
- Glaucoma (nerve damage in the eye)
- Round face (moon face) (Cushing's syndrome)
Your doctor will examine you periodically for these adverse effects and ensure that you are taking the lowest dose of this combination of drugs to control asthma.
- Irregular or rapid heartbeat or extra heartbeats (arrhythmias). Inform your doctor, but do not stop treatment with Seffalair Spiromax unless your doctor tells you to.
- A fungal infection of the esophagus, which can cause difficulty swallowing.
Frequency not known, but may also appear:
- Blurred vision.
Reporting Adverse Effects
If you experience any adverse effects, consult your doctor, pharmacist, or nurse, even if they are possible adverse effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of Seffalair Spiromax
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and on the inhaler label after CAD/EXP. The expiration date is the last day of the month indicated.
Do not store above 25°C. Keep the mouthpiece cover closed after removing the silver foil wrapper.
Use within 2 months of opening the silver foil wrapper.Use the label on the inhaler to note the date you opened the foil wrapper.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Seffalair Spiromax
- The active ingredients are salmeterol and fluticasone propionate. Each measured dose contains 14 micrograms of salmeterol (as salmeterol xinafoate) and 232 micrograms of fluticasone propionate. Each delivered dose (the dose that comes out of the mouthpiece) contains 12.75 micrograms of salmeterol (as salmeterol xinafoate) and 202 micrograms of fluticasone propionate.
- The other ingredient is lactose monohydrate (see section 2, "Seffalair Spiromax contains lactose").
Appearance and Package Contents
Each Seffalair Spiromax inhaler contains 60 inhalations of powder for inhalation and has a white body with a semi-transparent yellow mouthpiece cover.
Seffalair Spiromax is available in packs containing 1 inhaler and in multipacks containing 3 cartons, each with 1 inhaler inside. Not all pack sizes may be marketed in your country.
Marketing Authorization Holder
Teva B.V.
Swensweg 5,
2031 GA Haarlem,
Netherlands
Manufacturer
Norton (Waterford) Limited T/A Teva Pharmaceuticals Ireland
Unit 14/15, 27/35 & 301, IDA Industrial Park, Cork Road, Waterford, Ireland
Teva Operations Poland Sp. z o.o.
Mogilska 80 Str. 31-546 Kraków, Poland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tel/Tél: +32 3 820 73 73 | Lietuva UAB Teva Baltics Tel: +370 5 266 02 03 |
България Тева ФармаБългарияЕООД Тел: +359 2 489 95 85 | Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Tel/Tél: +32 3 820 73 73 |
Česká republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Magyarország Teva Gyógyszergyár Zrt Tel.: +36 1 288 64 00 |
Danmark Teva Denmark A/S Tlf: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland Ireland Tel: +44 207 540 7117 |
Deutschland Teva GmbH Tel: +49 731 402 08 | Nederland Teva Nederland B.V. Tel: +31 800 0228 400 |
Eesti UAB Teva Baltics Eesti filiaal Tel: +372 661 0801 | Norge Teva Norway AS Tlf: +47 6677 55 90 |
Ελλάδα Specifar A.B.E.E. Τηλ: +30 211 880 5000 | Österreich ratiopharm Arzneimittel Vertriebs GmbH Tel: +43 1 97007 0 |
España Teva Pharma S.L.U. Tél: +34 91 387 32 80 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel.: +48 22 345 93 00 |
France Teva Santé Tél: +33 1 55 91 7800 | Portugal Teva Pharma - Produtos Farmacêuticos Lda Tel: +351 21 476 75 50 |
Hrvatska Pliva Hrvatska d.o.o Tel: + 385 1 37 20 000 | România Teva Pharmaceuticals S.R.L Tel: +4021 230 6524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 207 540 7117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Ísland Teva Pharma Iceland ehf Simi: +354 550 3300 | Slovenská republika Teva Pharmaceuticals Slovakia s.r.o. Tel: +421 2 5726 7911 |
Italia Teva Italia S.r.l. Tel: +39 028 917 981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 20 180 5900 |
Κύπρος Specifar A.B.E.E. Ελλάδα Τηλ: +30 211 880 5000 | Sverige Teva Sweden AB Tel: +46 42 12 11 00 |
Latvija Teva Baltics filiale Latvija Tel: +371 673 23 666 | United Kingdom (Northern Ireland) Teva Pharmaceuticals IrelandIreland Tel: +44 207 540 7117 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Average pharmacy price41.28 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SEFFALAIR SPIROMAX 12.75 micrograms/202 micrograms INHALATION POWDERDosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/500 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms / 100 microgramsActive substance: salmeterol and fluticasoneManufacturer: Zentiva K.S.Prescription required
Online doctors for SEFFALAIR SPIROMAX 12.75 micrograms/202 micrograms INHALATION POWDER
Discuss questions about SEFFALAIR SPIROMAX 12.75 micrograms/202 micrograms INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















