
Airflusal Forspiro

Ask a doctor about a prescription for Airflusal Forspiro

How to use Airflusal Forspiro
Leaflet attached to the packaging: patient information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
AirFluSal Forspiro (Airflusan Forspiro)
(50 micrograms + 500 micrograms)/dose, powder for inhalation, divided
Salmeterol + Fluticasone propionate
AirFluSal Forspiro and Airflusan Forspiro are different trade names for the same medicine.
You should read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. It should not be passed on to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is AirFluSal Forspiro and what is it used for
- 2. Important information before using AirFluSal Forspiro
- 3. How to use AirFluSal Forspiro
- 4. Possible side effects
- 5. How to store AirFluSal Forspiro
- 6. Contents of the packaging and other information
1. What is AirFluSal Forspiro and what is it used for
AirFluSal Forspiro is used to treat:
- bronchial asthma
- chronic obstructive pulmonary disease (COPD). This disease is characterized by persistent breathing difficulties due to narrowed airways, often with coughing and excessive mucus production. The medicine reduces the frequency of COPD symptom exacerbations.
The medicine contains two active substances:
- salmeterol: a long-acting bronchodilator
- fluticasone: a corticosteroid that reduces swelling and inflammation in the lungs.
2. Important information before using AirFluSal Forspiro
When not to use AirFluSal Forspiro
- if the patient is allergicto salmeterol, fluticasone or to any of the excipients of the medicine (listed in section 6).
Warnings and precautions
Before using AirFluSal Forspiro, you should discuss it with your doctor or pharmacist,
if you have:
- heart disease, including irregular or rapid heartbeat
- hyperthyroidism
- high blood pressure
- diabetes (AirFluSal Forspiro may increase blood sugar levels)
- low potassium levels in the blood
- tuberculosis (currently or in the past) or other lung infections.
If the patient starts to see blurred vision or has other vision disturbances, they should tell their doctor.
AirFluSal Forspiro prevents the occurrence of shortness of breath and wheezing. It should be remembered,
that the medicine does not work when shortness of breath or wheezing has already occurred in the patient.
In case of an asthma attack, it is necessary to use a medicine (such as salbutamol) that
quickly expands the airways.
If the symptoms of asthma or breathing difficulties worsen, you should immediately inform your doctor. This may be manifested by:
- wheezing
- more frequent feeling of chest tightness
- greater than usual need for a fast-acting bronchodilator.
In such a case, you should continue using AirFluSal Forspiro, but not increase the number of inhalations, as the patient's condition may worsen, leading to severe illness. You should contact your doctor, as additional treatment may be necessary.
Children and adolescents
AirFluSal Forspiro should not be used in children and adolescents under 18 years of age.
AirFluSal Forspiro and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription.
AirFluSal Forspiro and the following medicines may interact with each other:
- medicines used to treat high blood pressure, heart disease or other diseases, containing an active substance ending in "-olol" (beta-adrenolytics), such as atenolol, propranolol and sotalol
- medicines used to treat viral infections, including some medicines used to treat HIV infection, such as ritonavir or cobicistat. The doctor may want to closely monitor the condition of the patient taking such medicines
- medicines used to treat infections, such as ketoconazole, itraconazole and erythromycin
- corticosteroids given orally or by injection: medicines used to treat inflammation or prevent transplant rejection
- diuretics used to treat high blood pressure
- other bronchodilators (such as salbutamol)
- medicines containing xanthine derivatives (often used to treat asthma).
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. The doctor will assess whether it is advisable to use AirFluSal Forspiro during pregnancy.
Driving and using machines
It is unlikely that AirFluSal Forspiro will affect your ability to drive or use machines.
AirFluSal Forspiro contains lactose monohydrate
If you have been diagnosed with an intolerance to some sugars, you should contact your doctor before taking the medicine.
The amount of lactose in the medicine usually does not cause any problems in people with lactose intolerance.
3. How to use AirFluSal Forspiro
This medicine should always be used in accordance with the doctor's recommendations. In case of doubts, you should consult a doctor or pharmacist.
Asthma
The recommended dose for adultsis:
one inhalation twice a day.
The doctor will regularly examine the patient for asthma symptoms.
COPD
The recommended dose for adultsis:
one inhalation twice a day.
If the symptoms have been controlled with AirFluSal Forspiro used twice a day, the doctor may reduce the dose to once a day:
- once in the evening, if the symptoms occur in the patient at night
- once in the morning, if the symptoms occur in the patient during the day.
Method of use
AirFluSal Forspiro should be used daily, as recommended by the doctor, preferably immediately before a mealin the morning and (or) evening.
After inhalation, the mouth should be rinsed with water.
Incorrect or non-compliant use of AirFluSal Forspiro may cause worsening of respiratory symptoms. To achieve optimal effectiveness, the medicine should be used daily, even if symptoms do not occur.
Instructions for use
The doctor, nurse or pharmacist should show how to use the inhaler and regularly check the correctness of its use.
The inhaler contains 60 doses of powder for inhalation in a rolled-up blister pack (strip) with foil. It is equipped with a dose counter that indicates how many doses are left (from 60 to 0). The numbers indicating the last 10 doses are placed on a red background.
The inhaler is not intended for refilling – after emptying, it should be replaced with a new device.
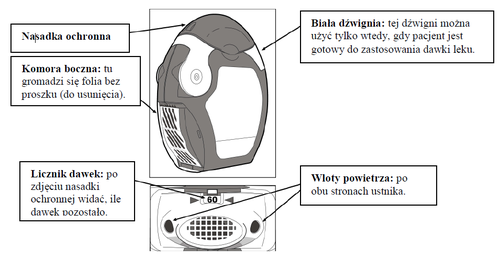
Before using the inhaler
- Open the transparent side chamber cover.
- Carefully tear off the entire length of the strip, using the notched edge (as shown in the picture). The strip should not be pulled or jerked.
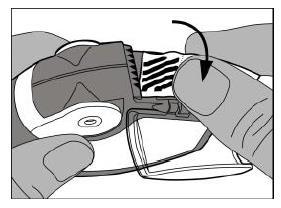
- Close the side chamber cover and discard the used strip of foil.
Warning:
As the inhaler is used, the side chamber gradually fills with the used strip of foil.
The strips of foil with black lines do not contain the medicine. At the end, numbered sections of the strip will appear in the chamber.
Do not allow more than 2 strips to be in the side chamber, as this may cause the inhaler to clog. Carefully tear off the strip of foil (as shown above) and dispose of it safely.
Using the inhaler
The inhaler should be held in the hands as shown in the pictures.
1. Opening
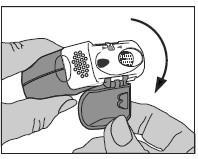
- Exposing the mouthpiece by pulling down the protective cap.
- Checking in the dose counter window how many doses of the medicine are left.
2. Preparing the dose of the medicine
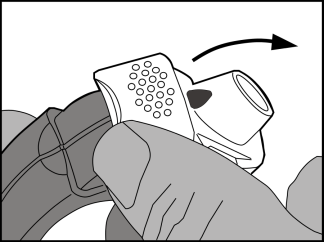
- Raisingthe edge of the white lever. Make sure the side chamber is closed. Warning: the white lever should only be used when the patient is ready to take the dose of the medicine. Unnecessary use of the lever causes loss of doses of the medicine.
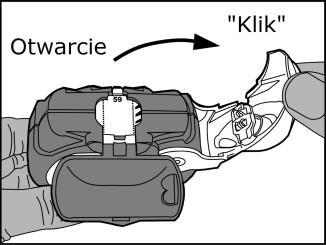
- Opening: pulling the white leverto the stop (audible click), which loads the dose of the medicine and displays its number in the dose counter.
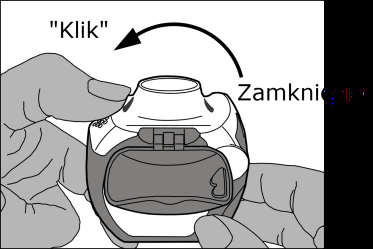
- Closing:carefully closingthe white lever so that an audible clickis heard. The inhaler is ready for immediate use.
3. Inhaling the dose of the medicine
- Exhaling as deeply as possible away from the mouthpiece. Never exhale into the inhaler, as this may change the size of the dose of the medicine.
- Holding the inhaler with the protective cap facing downwards.
- Tightly closing the mouthpiece with the lips.
- Taking a calm, deep breaththrough the mouth (not the nose).
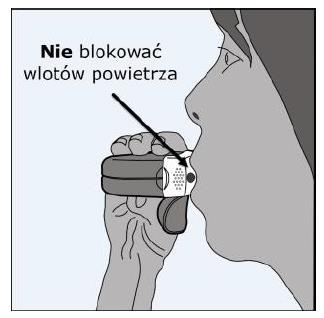
- Removing the inhaler from the mouth and holding the breath for 5-10 secondsor for as long as possible without feeling discomfort.
- Then exhaling slowly not in the direction of the inhaler.
- Closing the protective cap of the mouthpiece.
- Rinsing the mouth with water (water should be spat out), which helps prevent the development of fungal infections in the mouth and the occurrence of hoarseness.
Cleaning
- If necessary, the outer part of the mouthpiece can be wiped with a clean, dry cloth.
- The inhaler should not be disassembled for cleaning or for any other purpose!
- The parts of the inhaler should not be cleaned with water or wet wipes, as moisture may change the size of the dose of the medicine!
- Never insert a needle or other sharp objects into the mouthpiece or other parts of the inhaler, as this may damage it!
Using a higher dose of AirFluSal Forspiro than recommended
You should contact a doctor or pharmacist.
Symptoms of overdose are:
- dizziness
- headache
- rapid heartbeat
- muscle weakness
- joint pain
- feeling of trembling.
If higher doses are used for a long time, you should consult a doctor or pharmacist for advice, as higher doses of AirFluSal Forspiro may cause a decrease in the production of steroid hormones by the adrenal glands.
It is important to use the medicine in accordance with the doctor's recommendations. You should not increase or decrease the dose without the doctor's consent.
Missing a dose of AirFluSal Forspiro
You should not take a double dose of the medicine to make up for a missed dose. The next dose of the medicine should be taken at the usual time.
Stopping the use of AirFluSal Forspiro
You should not stop using AirFluSal Forspiro or suddenly reduce the dose of the medicine without the doctor's consent, as this may worsen breathing disorders and very rarely cause side effects, such as:
- abdominal pain
- feeling of fatigue and loss of appetite, nausea
- vomiting and diarrhea
- weight loss
- headache or drowsiness
- low blood sugar levels
- low blood pressure and seizures.
Similar side effects may also occur very rarely during infection or in a situation of severe stress (e.g. due to fever, injury, serious accident or surgery). To prevent these symptoms, the doctor may prescribe additional corticosteroids (e.g. prednisolone) to the patient.
In case of any further doubts related to the use of this medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions: the patient may experience sudden breathing difficulties immediately after
using AirFluSal Forspiro.Wheezing, coughing or shortness of breath may occur, as well as itching, rash (hives) and swelling (usually of the face, lips, tongue or throat), as well as a feeling of a very rapid heartbeat or fainting and dizziness (which may lead to a fall or loss of consciousness). If any of these symptoms occur or if they occur suddenly after using AirFluSal Forspiro, you should immediately inform your doctor.Allergic reactions to AirFluSal Forspiro are uncommon (they may occur less often than in 1 in 100 people).
Other possible side effects:
Very common(may occur more often than in 1 in 10 people)
- headache (usually resolving during treatment)
- increased number of colds in patients with chronic obstructive pulmonary disease (COPD).
Common(may occur less often than in 1 in 10 people)
- thrush (painful, creamy-yellow patches) in the mouth and throat, as well as tongue pain, hoarseness and throat irritation. Rinsing the mouth with water and immediately spitting it out may be helpful, and (or) brushing teeth immediately after each inhalation. The doctor may prescribe an antifungal medicine to treat thrush
- pain, swelling of the joints and muscle pain
- muscle cramps.
In patients with chronic obstructive pulmonary disease (COPD), the following side effects have been reported:
- pneumonia (lung infection). You should tell your doctor if any of the following symptoms occur while using AirFluSal Forspiro; these may be symptoms of a lung infection:
- increased mucus production
- change in the color of the mucus
- fever
- chills
- worsening cough
- increased breathing difficulties
- bruising and fractures
- sinusitis
- low potassium levels in the blood (which may cause irregular heartbeat, weakness and (or) muscle cramps).
Uncommon(may occur less often than in 1 in 100 people)
- very rapid heartbeat (tachycardia)
- feeling of trembling and rapid or irregular heartbeat (palpitations). These are usually harmless symptoms that resolve during treatment.
- feeling of sadness (occurring mainly in children)
- increased blood sugar levels. In patients with diabetes, it may be necessary to monitor blood sugar levels more frequently and adjust the dose of the antidiabetic medicine
- sleep disturbances
- chest pain
- cataract (clouding of the lens)
- allergic rash.
Rare(may occur less often than in 1 in 1000 people)
- breathing difficultiesor wheezingthat worsensimmediately after using AirFluSal Forspiro. You should stop usingAirFluSal Forspiro and immediately inform your doctor.To facilitate breathing, a fast-acting bronchodilator should be used in an inhalation
- abnormal production of certain hormones, especially if the medicine is used in high doses for a long time. Symptoms are: slowed growth in children and adolescents, osteoporosis, increased eye pressure (glaucoma)
weight gain
rounded ( "moon-shaped") face (Cushing's syndrome).
The doctor will regularly check if the patient has any of the above side effects and ensure that the patient is using the smallest possible dose of the medicine.
- changes in behavior, such as unusual agitation and irritability. Such symptoms occur mainly in children
- irregular heartbeat or extra heartbeats. You should tell your doctor about this, but do not stop using AirFluSal Forspiro without a doctor's recommendation
- fungal infection of the esophagus, which may cause difficulty swallowing.
Frequency not known, but may also occur:
- depression or aggression. Such symptoms occur mainly in children
- blurred vision.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help gather more information on the safety of the medicine.
5. How to store AirFluSal Forspiro
The medicine should be stored out of sight and reach of children.
Store in a temperature below 25°C.
Do not use AirFluSal Forspiro after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer used. This will help protect the environment.
6. Contents of the packaging and other information
What AirFluSal Forspiro contains
- The active substances of the medicine are salmeterol and fluticasone propionate. Each dose contains 50 micrograms of salmeterol (as salmeterol xinafoate) and 500 micrograms of fluticasone propionate. This corresponds to a delivered dose containing 45 micrograms of salmeterol (as salmeterol xinafoate) and 465 micrograms of fluticasone propionate.
- The other ingredient is lactose monohydrate.
What AirFluSal Forspiro looks like and what the pack contains
An inhaler with a blister pack of foil containing 60 doses of powder for inhalation.
Pack sizes: 1 or 2 inhalers containing 60 doses each.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in the Czech Republic, the country of export:
Sandoz s.r.o.
Na Pankráci 1724/129
140 00 Prague 4 – Nusle
Czech Republic
Manufacturer:
Aeropharm GmbH
Francois-Mitterand-Allee 1
Wohngebiet Rudolspark
07407 Rudolstadt
Thuringia, Germany
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in the Czech Republic, the country of export:14/412/14-C
Parallel import authorization number: 199/25
Date of leaflet approval: 13.06.2025
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Sandoz s.r.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Airflusal ForspiroDosage form: Powder, 50 mcg + 250 mcgActive substance: salmeterol and fluticasoneManufacturer: Aeropharm GmbHPrescription requiredDosage form: Powder, 50 mcg + 500 mcgActive substance: salmeterol and fluticasoneManufacturer: Aeropharm GmbHPrescription requiredDosage form: Powder, (50 micrograms + 250 micrograms)/doseActive substance: salmeterol and fluticasonePrescription required
Alternatives to Airflusal Forspiro in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Airflusal Forspiro in Spain
Alternative to Airflusal Forspiro in Ukraine
Online doctors for Airflusal Forspiro
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Airflusal Forspiro – subject to medical assessment and local rules.














