
AMAIRA 50 micrograms/100 micrograms Inhalation Powder (single dose)

How to use AMAIRA 50 micrograms/100 micrograms Inhalation Powder (single dose)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
Amaira 50micrograms/100micrograms/inhalation, powder for inhalation (single-dose)
Amaira 50micrograms/250micrograms/inhalation, powder for inhalation (single-dose)
salmeterol/fluticasone propionate
Read this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Amaira is and what it is used for
- What you need to know before you use Amaira
- How to use Amaira
- Possible side effects
- Storing Amaira
- Contents of the pack and further information
1. What Amaira is and what it is used for
Amaira contains two active substances, salmeterol and fluticasone propionate:
- Salmeterol is a long-acting bronchodilator. Bronchodilators help to keep the airways in the lungs open, making it easier to breathe in and out. The effects last for at least 12 hours.
- Fluticasone propionate is a corticosteroid that reduces inflammation and irritation in the lungs.
This medicine is used to treat adults and adolescents from 12 years of age.
Your doctor has prescribed this medicine to help prevent breathing problems such as:
- Asthma
You should use Amaira every day, as your doctor has recommended. This will ensure that the medicine works correctly to control your asthma.
Salmeterol/fluticasonehelps to prevent shortness of breath and wheezing. However, Amaira should not be used to relieve a sudden attack of shortness of breath or wheezing. In such cases, you should use your "rescue" medication, such as salbutamol. You should always carry your "rescue" medication with you.
2. What you need to know before you use Amaira
Do not use Amaira
If you are allergic to salmeterol, fluticasone propionate, or any of the other ingredients, lactose monohydrate.
Warnings and precautions
Talk to your doctor or pharmacist before starting treatment with salmeterol/fluticasone if you have:
- Heart problems, including fast or irregular heartbeat
- Overactive thyroid gland
- High blood pressure
- Diabetes mellitus (salmeterol/fluticasone may increase blood sugar levels)
- Low levels of potassium in the blood
- Tuberculosis (TB) now or in the past, or other lung infections
Consult your doctor if you experience blurred vision or other visual disturbances.
- Children
- This medicine should not be used in children under 12 years of age.
Using Amaira with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This is because, in some cases, salmeterol/fluticasone may not be suitable to be taken with other medicines.
Inform your doctor if you are taking any of the following medicines before starting to use salmeterol/fluticasone:
- Beta-blockers (such as atenolol, propranolol, and sotalol). Beta-blockers are mainly used to treat high blood pressure or other heart conditions, such as angina.
- Medicines to treat infections (such as ketoconazole, itraconazole, and erythromycin), including some medicines for HIV (such as ritonavir, medicines containing cobicistat). Some of these medicines may increase the amount of fluticasone propionate or salmeterol in your body. This may increase your risk of experiencing side effects with Amaira, including irregular heartbeats, or may worsen side effects. Therefore, your doctor will monitor you closely if you are taking these medicines.
- Corticosteroids (oral or injectable). If you have taken these medicines recently, you may be at increased risk of salmeterol/fluticasone affecting your adrenal glands.
- Diuretics, also known as water pills, used to treat high blood pressure.
- Other bronchodilators (such as salbutamol).
- Medicines containing xanthines, such as aminophylline or theophylline. These are often used to treat asthma.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Salmeterol/fluticasone is unlikely to affect your ability to drive or use machines.
Amairacontains lactose
Amaira contains approximately 13 milligrams of lactose in each dose. This amount normally does not cause problems in people with lactose intolerance. Lactose may contain milk proteins that can cause allergic reactions in patients with cow's milk protein allergy.
3. How to use Amaira
Follow the instructions for administration of this medicine exactly as prescribed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
- Use Amaira every day, until your doctor tells you to stop. Do not take more than the recommended dose. If you are unsure, consult your doctor or pharmacist.
- Do not stop taking this medicine or reduce the dose without talking to your doctor first.
- Salmeterol/fluticasone should be inhaled through the mouth into the lungs.
The recommended dose is:
Adults and adolescents from 12years of age and older
- Amaira 50/100 One inhalation twice a day
- Amaira 50/250 One inhalation twice a day
Your symptoms may be well-controlled using this medicine twice a day. If this is the case, your doctor may decide to reduce your dose to once a day. The dose may change to:
- once at night if you have night-timesymptoms,
- once in the morning if you have day-timesymptoms.
It is very important that you follow your doctor's instructions about how many applications and how often you should take your medicine.
If you are using Amaira to treat asthma, your doctor will want to regularly monitor your symptoms.
If your asthma gets worse or you have more difficulty breathing, see your doctor immediately. You may notice more wheezing or shortness of breath, or you may need to use your "rescue" medication more often. If any of these things happen, you should continue to use salmeterol/fluticasone, but do not increase the number of applications. Your lung disease may get worse, and you may become seriously ill. See your doctor, as you may need additional treatment.
Instructions for use
- Amaira may be different from the inhalers you have used in the past, so it is very important that you use it correctly. Your doctor, nurse, or pharmacist should teach you how to use your inhaler. This training is important to ensure that you receive the dose you need . If you have not received this training, ask your doctor, nurse, or pharmacist to show you how to use the inhaler correctly, especially if it is the first time you use it.
Periodically, they should check how you use it to ensure that you are using the device correctly as prescribed. Not using Amaira properly or as prescribed may result in your asthma not improving as it should.
- This device contains blisters that contain salmeterol/fluticasone propionate in powder form.
- There is a dose counter on the top of the device that shows how many doses are left. It counts down to 0. The numbers from 5 to 0 appear in red to warn you that there are few doses left. Once the counter reaches 0, your inhaler is empty.
Using your inhaler
- To open the inhaler, hold it with one hand in a flat position. Press the red button with your thumb (see figure 1) and slide the light pink cap (for 50/100 micrograms) or pink cap (for 50/250 micrograms) away from you as far as possible with the thumb of the other hand until you hear a "click" (see figure 2). This will open a small hole in the mouthpiece, and place a dose of medicine in the mouthpiece.
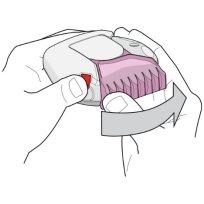
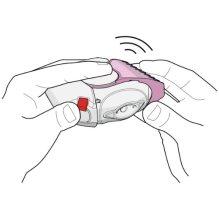
Figure 1 Figure 2
Be aware that each time you open the mouthpiece cap with a "click", a blister is opened, and the powder is prepared for inhalation. Therefore, do not open the mouthpiece cap if you do not need to take the medicine, as the blisters will open, and medicine will be wasted.
- Hold the inhaler away from your mouth. Breathe out as much as you can. Do not breathe into the inhaler.
- Place the mouthpiece in your lips (see figure 3). Take a slow, deep breath in through the inhaler, not through your nose.
Take the inhaler out of your mouth.
Hold your breath for about 10 seconds or as long as you can.
Breathe out slowly.
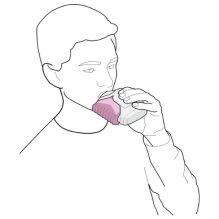
Figure 3
The inhaler releases its dose of medicine in the form of a very fine powder. You may not be able to taste or feel the powder. Do not use an additional dose of the inhaler if you do not taste or feel the medicine.
- Close the inhaler to keep it clean, sliding the light pink cap (for 50/100 micrograms) or pink cap (for 50/250 micrograms) back to its original position. You will hear a "click" (seefigure4). The mouthpiece cap returns to its original position and is reset. The inhaler is now ready for you to take your next scheduled dose.
- Rinse your mouth with water and spit it out, or brush your teeth. This may help prevent mouth ulcers and hoarseness.
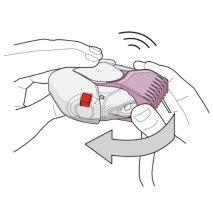
Figure 4
Cleaning your inhaler
Keep your inhaler clean and dry.
To clean it, wipe the mouthpiece of the inhaler with a dry cloth.
If you use more Amaira than you should
It is very important to use the inhaler exactly as prescribed. If you accidentally take more doses than recommended, consult your doctor or pharmacist. You may notice that your heart beats faster than normal, and you may feel shaky. You may also feel dizzy, have a headache, weakness, and joint pain.
If you have used high doses of salmeterol/fluticasone for long periods, you should ask your doctor or pharmacist for advice. This is because using high concentrations of this medicine may reduce the amount of steroid hormones produced by the adrenal glands.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use Amaira
Do not take a double dose to make up for forgotten doses. Take the next dose at the usual time.
If you stop using Amaira
It is very important that you use salmeterol/fluticasone every day as prescribed. Continue to take it until your doctor tells you to stop. Do not stop or suddenly reduce your treatment withsalmeterol/fluticasone.This could make your breathing worse.
Additionally, if you stop taking salmeterol/fluticasone suddenly or reduce your dose, you may (very rarely) experience problems with your adrenal glands (adrenal insufficiency), which can sometimes cause side effects.
These side effects may include any of the following:
- Stomach pain
- Tiredness and loss of appetite, feeling unwell
- Discomfort and diarrhea
- Weight loss
- Headache or drowsiness
- Low blood sugar levels
- Low blood pressure and seizures (fits)
When your body is under stress, such as fever, trauma (e.g., a car accident or injury), infection, or surgery, adrenal insufficiency may worsen, and you may experience any of the above side effects.
If you experience any side effects, consult your doctor or pharmacist. To prevent these symptoms, your doctor may prescribe an additional dose of corticosteroids in tablets (such as prednisolone).
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. To reduce the appearance of adverse effects, your doctor will prescribe the lowest dose of this combination of medicines that controls your asthma.
Allergic Reactions: you may notice that your breathing suddenly worsens immediately after using Amaira.You may suffer from wheezing and coughing or shortness of breath. You may also notice itching, rash (urticaria), and swelling (usually of the face, lips, tongue, or throat). You may also suddenly feel that your heart is beating very fast, feel like you are losing consciousness, and feel dizzy (which can lead to collapse or loss of consciousness). If you experience any of these effects or if they appear suddenly after using Amaira, stop takingthis medicineand inform your doctor immediately.Allergic reactions to Amaira are rare (they can affect up to 1 in 100 people).
Very Common (may affect more than 1 in 10people)
- Headache, which usually improves as treatment continues.
Common (may affect up to 1 in 10people)
- Thrush (itching, appearance of yellowish-white ulcers) in the mouth and throat. Also, pain in the tongue, hoarse voice, and throat irritation. Rinsing your mouth with water and spitting it out and/or brushing your teeth immediately after each dose of medicine may help. For the treatment of thrush, your doctor may prescribe antifungal medication (for the treatment of fungal infections).
- Pain, inflammation in the joints, and muscle pain.
- Muscle cramps.
Uncommon (may affect up to 1 in 100people)
- Increased blood sugar (glucose) levels (hyperglycemia). If you have diabetes, you will need to check your blood sugar levels more frequently and adjust your usual diabetic treatment if necessary.
- Cataracts (opacity of the eye lens).
- Very fast heart rate (tachycardia).
- Feeling tremors and a fast or irregular heart rate (palpitations). These adverse effects are usually harmless and decrease as treatment continues.
- Chest pain.
- Feeling of anxiety (occurs mainly in children).
- Sleep disorders.
- Allergic skin rash.
Rare (may affect up to 1 in 1,000people)
- Difficulty breathing or wheezing that worsens immediately after using Amaira. Ifthis happens, stop using the Amaira inhaler. Use your rapid-acting "rescue" inhaler to improve your breathing and inform your doctor immediately.
- Salmeterol/fluticasone may increase the normal production of steroid hormones, particularly if you have been taking high doses for long periods. The effects include:
- Growth delay in children and adolescents
- Decreased bone mineral density
- Glaucoma
- Weight gain
- Round face (moon face) (Cushing's syndrome).
Your doctor will regularly monitor you for the appearance of any of these adverse effects and ensure that you are taking the lowest dose of this combination of medicines to control your asthma.
- Changes in behavior, such as hyperactivity and irritability (although these effects occur mainly in children).
- Uneven or irregular heartbeats or extra heartbeats (arrhythmias). Consult your doctor, but do not stop taking Amaira unless your doctor tells you to do so.
- Fungal infection in the esophagus (throat), which can cause difficulty swallowing.
Frequency not known, but may also appear:
- Depression or aggression (it is more likely that these effects will appear in children).
- Blurred vision
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Amaira
- Keep this medicine out of the sight and reach of children.
- Do not use Amaira after the expiration date that appears on the packaging and on the label of your inhaler after CAD. The expiration date is the last day of the month indicated.
- Do not store at a temperature above 30 °C.
- Medicines should not be thrown away through drains or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Amaira
The active ingredients are salmeterol and fluticasone propionate.
Amaira 50 micrograms/100 micrograms
Each individual inhalation provides a released dose (the dose that comes out of the mouthpiece) of 47 micrograms of salmeterol (as salmeterol xinafoate) and 92 micrograms of fluticasone propionate. This corresponds to a pre-dispensed dose of 50 micrograms of salmeterol (as salmeterol xinafoate) and 100 micrograms of fluticasone propionate.
Amaira 50 micrograms/250 micrograms
Each individual inhalation provides a released dose (the dose that comes out of the mouthpiece) of 45 micrograms of salmeterol (as salmeterol xinafoate) and 229 micrograms of fluticasone propionate. This corresponds to a pre-dispensed dose of 50 micrograms of salmeterol (as salmeterol xinafoate) and 250 micrograms of fluticasone propionate.
The other component is lactose monohydrate (see section 2 in "Amaira contains lactose") (contains milk proteins).
Appearance of the Product and Package Contents
- Amaira contains a strip of alveoli filled with white to off-white powder. The aluminum paper protects the inhalation powder from atmospheric effects.
- Each dose is pre-dispensed.
- The white plastic devices with a light pink mouthpiece cover (for 50/100 micrograms), or pink (for 50/250 micrograms) are found in packages of:
1, 2, 3, or 10 x inhalers with 60 inhalations each.
Not all package sizes may be marketed.
Marketing Authorization Holder:
Zentiva k.s.
U Kabelovny 130
102 37 Prague 10
Czech Republic
Manufacturer:
Oy Medfiles Ltd
Volttikatu 5, Volttikatu 8
Kuopio, 70700
Finland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder: Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Spain
This medicine is authorized in the Member States of the European Economic Area with the following names:
Austria | Everio Airmaster |
Belgium | Brecur Airmaster 50 microgram/100 microgram/dose, inhalatiepoeder, voorverdeeld Brecur Airmaster 50 microgram/250 microgram/dose, inhalatiepoeder, voorverdeeld Brecur Airmaster 50 microgrammes/100 microgrammes/dose, poudre pour inhalation en récipient unidose Brecur Airmaster 50 microgrammes/250 microgrammes/dose, poudre pour inhalation en récipient unidose Brecur Airmaster 50 Mikrogramm/100 mikrogramm einzeldosiertes Pulver zur Inhalation Brecur Airmaster 50 Mikrogramm/250 Mikrogramm einzeldosiertes Pulver zur Inhalation |
Bulgaria | ?????? ????????? 50 ??????????/100 ??????????/???? ???? ?? ?????????, ????????????? ??????? ?????? ????????? 50 ??????????/250 ??????????/???? ???? ?? ?????????, ????????????? ??????? |
Slovakia | Everio Airmaster 50 mikrogramov/100 mikrogramov Everio Airmaster 50 mikrogramov/250 mikrogramov |
Spain | Amaira 50 micrograms/100 micrograms/inhalation, powder for inhalation (unidose) Amaira 50 micrograms/250 micrograms/inhalation, powder for inhalation (unidose) |
Estonia | Everio Airmaster |
France | PROPIONATE DE FLUTICASONE/SALMETEROL ZENTIVA 100 microgrammes/50 microgrammes/dose, poudre pour inhalation en récipient unidose PROPIONATE DE FLUTICASONE/SALMETEROL ZENTIVA 250 microgrammes/50 microgrammes/dose, poudre pour inhalation en récipient unidose |
Hungary | Fluzalto Airmaster 50 mikrogramm/100 mikrogramm/adag adagolt inhalációs por Fluzalto Airmaster 50 mikrogramm/250 mikrogramm/adag adagolt inhalációs por |
Ireland | Bronx Airmaster |
Latvia | Everio Airmaster 50/100 mikrogrami/deva inhalacijas pulveris, dozets Everio Airmaster 50/250 mikrogrami/deva inhalacijas pulveris, dozets |
Lithuania | Everio Airmaster 50/100 mikrogramu/dozeje dozuoti ikvepiamieji milteliai Everio Airmaster 50/250 mikrogramu/dozeje dozuoti ikvepiamieji milteliai. |
Poland | Neuair Airmaster |
Czech Republic | Everio Airmaster |
Romania | Everio Airmaster 50 micrograme /100 micrograme pulbere de inhalat unidoza Everio Airmaster 50 micrograme /250 micrograme pulbere de inhalat unidoza |
Sweden | Neuair Airmaster |
Date of the last revision of this prospectus:November 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/.
- Country of registration
- Average pharmacy price41.28 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMAIRA 50 micrograms/100 micrograms Inhalation Powder (single dose)Dosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/500 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Zentiva K.S.Prescription required
Online doctors for AMAIRA 50 micrograms/100 micrograms Inhalation Powder (single dose)
Discuss questions about AMAIRA 50 micrograms/100 micrograms Inhalation Powder (single dose), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















