ROTATEQ ORAL SOLUTION


How to use ROTATEQ ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
RotaTeq Oral Solution
Rotavirus vaccine (live virus)
Read all of this leaflet carefully before your child is vaccinated because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If your child experiences any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is RotaTeq and what is it used for
- What you need to know before your child receives RotaTeq
- How to use RotaTeq
- Possible side effects
- Storage of RotaTeq
- Contents of the pack and other information
1. What is RotaTeq and what is it used for
RotaTeq is an oral vaccine that helps protect infants and young children from gastroenteritis (diarrhea and vomiting) caused by rotavirus infection and can be given to children from 6 weeks up to 32 weeks (see section 3). The vaccine contains five strains of live rotavirus. When the vaccine is given to a child, the immune system (the body's natural defenses) will create antibodies against the types of rotavirus that occur most frequently. These antibodies help protect against gastroenteritis caused by these types of rotavirus.
2. What you need to know before your child receives RotaTeq
Do not use RotaTeq if
- your child is allergic to any of the components of this vaccine (see section 6. Contents of the pack and other information).
- your child developed an allergic reaction after receiving a dose of RotaTeq or another rotavirus vaccine.
- your child has had a previous intussusception (a bowel obstruction in which a segment of intestine slides into another segment).
- your child was born with a gastrointestinal malformation that could predispose to intussusception.
- your child has any disease that reduces their resistance to infection.
- your child has a severe infection with high fever. Vaccination may need to be postponed until recovery. A mild infection such as a cold should not be a problem, but consult your doctor first.
- your child has diarrhea or vomiting. Vaccination may need to be postponed until recovery.
Warnings and precautions
Consult your doctor or pharmacist before starting to use RotaTeq, if your child:
- has received a blood transfusion or immunoglobulins in the last 6 weeks.
- has close contact with someone, such as a family member, who has a weakened immune system, for example, a person with cancer or taking medicines that can weaken the immune system.
- has any gastrointestinal disorder.
- has not been gaining weight and growing as expected.
- or the mother has received any medicine during pregnancy that weakens the immune system.
If after administration of RotaTeq your child experiences severe stomach pain, persistent vomiting, blood in the stool, swollen abdomen and/or high fever, contact a doctor/healthcare professional immediately (see also section 4 “Possible side effects”).
As always, be careful to wash your hands thoroughly after changing used diapers.
As with other vaccines, RotaTeq does not guarantee complete protection in all vaccinated children, even after the three doses have been given.
If your child has already been infected with rotavirus but was not yet ill when vaccinated, RotaTeq may not prevent the disease.
RotaTeq does not protect against diarrhea and vomiting due to causes other than rotavirus.
Other medicines and RotaTeq
RotaTeq can be given at the same time as your child receives other vaccinations normally recommended, such as vaccines against diphtheria, tetanus, pertussis (whooping cough), Haemophilus influenzaetype b, inactivated or oral poliomyelitis, hepatitis B, pneumococcal conjugate vaccine, and meningococcal conjugate vaccine.
Tell your doctor or pharmacist if your child is using, has recently used, or might use any other medicines (or other vaccines).
Using RotaTeq with food and drinks
There are no restrictions on food or drink consumption, including breast milk, before or after vaccination with RotaTeq.
RotaTeq contains sucrose
If your child has been told that they have an intolerance to some sugars, inform your doctor or healthcare professional before the vaccine is given.
RotaTeq contains sodium
This vaccine contains 37.6 mg of sodium (the main component of table/cooking salt) per dose. This is equivalent to 1.88% of the maximum recommended daily sodium intake for an adult.
3. How to use RotaTeq
RotaTeq IS ADMINISTERED ONLY ORALLY.
A doctor or nurse will give your child the recommended doses of RotaTeq. The vaccine will be given by carefully squeezing the tube and administering the vaccine into your child's mouth. The vaccine can be given with or without food, drink, or breast milk. If your child spits up or regurgitates most of the vaccine dose, a single replacement dose can be given at the same vaccination visit.
This vaccine should not be injected under any circumstances.
The first dose (2 ml) of RotaTeq can be given from 6 weeks of age and must be given before 12 weeks of age (about 3 months). RotaTeq can be given to premature infants as long as the pregnancy lasted at least 25 weeks. These children should receive the first dose of the vaccine between 6 and 12 weeks after birth.
Your child will receive 3 doses of RotaTeq, given at least 4 weeks apart. It is essential that your child receives all 3 doses of the vaccine to be protected against rotavirus. It is recommended that the three doses be given before 20-22 weeks of age and no later than 32 weeks of age.
When RotaTeq is given to your child for the first dose, it is recommended to continue with RotaTeq (and not with another rotavirus vaccine) to complete the vaccination regimen.
If you miss a dose of RotaTeq
It is essential that you follow your doctor's/healthcare professional's instructions regarding your child's follow-up visits for the next doses. If you miss or cannot attend your appointment on the scheduled date, consult your doctor/healthcare professional.
4. Possible side effects
Like all vaccines and medicines, this vaccine can cause side effects, although not everybody gets them.
Contact a doctor/healthcare professional immediately if your child experiences any of the following symptoms:
- Allergic reactions (frequency cannot be estimated from the available data), which can be severe (anaphylaxis), and can include: allergic swelling that can affect the face, lips, tongue, or throat.
- Bronchospasm (rare, may affect up to 1 in 1,000 children): This may present as wheezing, coughing, or difficulty breathing.
- Severe stomach pain, persistent vomiting, blood in the stool, swollen abdomen, and/or high fever. These could be symptoms of a very rare (may affect up to 1 in 10,000 children) but serious side effect called intussusception (a bowel obstruction in which a segment of intestine slides into another segment).
The following side effects have been reported with the use of RotaTeq:
- Very common (may affect more than 1 in 10 children): fever, diarrhea, vomiting
- Common (may affect up to 1 in 10 children): upper respiratory tract infections
- Uncommon (may affect up to 1 in 100 children): stomach pain (see also the previous paragraph for signs of the very rare side effect intussusception), runny nose and sore throat, ear infection, rash, blood in the stool
- Rare (may affect up to 1 in 1,000 children): hives
- Frequency not known (cannot be estimated from the available data): irritability
In children born very prematurely (at 28 weeks of gestation or earlier), longer-than-normal pauses between breaths may occur during the 2 or 3 days following vaccination.
If you want more information about the side effects of RotaTeq, talk to your doctor or healthcare professional.
Reporting of side effects
If your child experiences any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of RotaTeq
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the label after EXP. (CAD). The expiry date is the last day of the month stated.
Store and transport refrigerated (between 2°C and 8°C). Keep the dosing tube in the outer packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What is in RotaTeq
The active substances in RotaTeq are 5 strains of human-bovine rotavirus reassortants:
G1 2.2 x 10^6 Infectious Units
G2 2.8 x 10^6 Infectious Units
G3 2.2 x 10^6 Infectious Units
G4 2.0 x 10^6 Infectious Units
P1A[8] 2.3 x 10^6 Infectious Units
The other ingredients in RotaTeq are: sucrose, sodium citrate, sodium dihydrogen phosphate monohydrate, sodium hydroxide, polysorbate 80, culture medium (which contains inorganic salts, amino acids, and vitamins) and purified water.
Appearance and packaging
Oral solution
This vaccine is contained in a single-dose tube and is a pale yellow, clear liquid that may have a pink tint.
RotaTeq is available in packs of 1 and 10 dosing tubes. Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, Netherlands
Manufacturer: Merck Sharp and Dohme, B.V., Waarderweg, 39, 2031 BN, Haarlem, Netherlands
You can get more information on this medicine by contacting the local representative of the marketing authorisation holder.
Belgium MSD Belgium Tel: +32(0)27766211 | Lithuania UAB Merck Sharp & Dohme Tel: +370 5 2780 247 |
| Luxembourg MSD Belgium Tel: +32(0)27766211 |
Czech Republic Merck Sharp & Dohme s.r.o. Tel.: +420 233 010 111 | Hungary MSD Pharma Hungary Kft. Tel.: + 36 1 888 5300 |
Denmark MSD Danmark ApS Tlf.: + 45 4482 4000 | Malta Merck Sharp & Dohme Cyprus Limited. Tel: 8007 4433 (+356 99917558) |
Germany MSD Sharp & Dohme GmbH Tel.: +49 (0) 89 20 300 4500 | Netherlands Merck Sharp & Dohme B.V. Tel: 0800 9999000 (+31 23 5153153) |
Estonia Merck Sharp & Dohme OÜ Tel: +372 614 4200 | Norway MSD (Norge) AS Tlf: +47 32 20 73 00 |
Greece MSD Α.Φ.Ε.Ε. Τηλ: +30 210 98 97 300 | Austria Merck Sharp & Dohme Ges.m.b.H. Tel: +43 (0) 1 26 044 |
Spain Merck Sharp & Dohme de España, S.A. Tel: +34 91 321 06 00 | Poland MSD Polska Sp. z o.o. Tel.: +48 22 549 51 00 |
France MSD France Tél: + 33 (0)1 80 46 40 40 | Portugal Merck Sharp & Dohme, Lda Tel: +351 21 4465700 |
Croatia Merck Sharp & Dohme d.o.o. Tel: +385 1 66 11 333 | Romania Merck Sharp & Dohme Romania S.R.L. Tel: + 40 21 529 29 00 |
Ireland Merck Sharp & Dohme Ireland (Human Health) Limited Tel: +353 (0)1 2998700 | Slovenia Merck Sharp & Dohme, inovativna zdravila d.o.o. Tel: +386 1 520 4201 |
Iceland Vistor ehf. Sími: + 354 535 7000 | Slovakia Merck Sharp & Dohme, s. r. o. Tel: +421 2 58282010 |
Italy MSD Italia S.r.l. Tel: 800 23 99 89 (+39 06 361911) | Finland MSD Finland Oy Puh/Tel: +358 (0)9 804 650 |
Cyprus Merck Sharp & Dohme Cyprus Limited Τηλ: 800 00 673 (+357 22866700) | Sweden Merck Sharp & Dohme (Sweden) AB Tel: +46 77 5700488 |
Latvia SIA Merck Sharp & Dohme Latvija Tel.: + 371 67025300 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Instructions
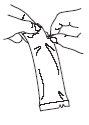
To administer the vaccine: | |
| Tear the protective bag and remove the dosing tube. |
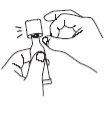
| Remove the liquid from the dispensing tip by holding the tube vertically and tapping the cap. |
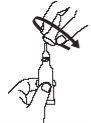
| Open the dosing tube with 2 simple movements:
|
|
|
| Administer the dose by carefully pouring the liquid into the inside of the child's mouth towards the inner cheek until the dosing tube is empty. (A residual drop may remain in the tip of the tube.) |
Discard the empty tube and cap in approved biological waste containers in accordance with local requirements. |
The unused medicine and all materials that have come into contact with it should be disposed of in accordance with local regulations.
See also section 3. How to use RotaTeq.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROTATEQ ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 10 million germsActive substance: rota virus, live attenuatedManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 10 million germsActive substance: rota virus, live attenuatedManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 10 MILLION GERMSActive substance: rota virus, live attenuatedManufacturer: Glaxosmithkline BiologicalsPrescription required
Online doctors for ROTATEQ ORAL SOLUTION
Discuss questions about ROTATEQ ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions