
ROACTEMRA 162 mg Injectable Solution in Pre-filled Syringe

How to use ROACTEMRA 162 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
RoActemra 162 mg, solution for injection in pre-filled syringe
tocilizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
In addition to this leaflet, you will be given a patient alert card, which contains important safety information that you need to know before you receive RoActemra and during treatment with RoActemra.
Contents of the pack:
- What is RoActemra and what is it used for
- What you need to know before you use RoActemra
- How to use RoActemra
- Possible side effects
- Storage of RoActemra
- Contents of the pack and other information
1. What is RoActemra and what is it used for
RoActemra contains the active substance tocilizumab, which is a protein obtained from specific immune cells (monoclonal antibody), that blocks the action of a specific type of protein (cytokine) called interleukin-6. This protein is involved in inflammatory processes of the body, and blocking it can reduce inflammation. RoActemra is indicated for the treatment of:
- adultswith moderate to severe active rheumatoid arthritis (RA), which is an autoimmune disease, if previous treatments have not worked well.
- adultswith severe, active, and progressive rheumatoid arthritis (RA) who have not been previously treated with methotrexate.
RoActemra helps reduce the symptoms of RA such as pain and swelling in the joints and can also improve performance in daily tasks. RoActemra has been shown to decrease the progression of damage to the cartilage and bones of the joints caused by the disease and improve the ability to perform daily activities.
RoActemra is usually used in combination with another medication for RA called methotrexate. However, RoActemra can be administered alone if your doctor determines that methotrexate is not suitable.
- adults with a disease of the arteries called giant cell arteritis (GCA), caused by inflammation of the largest arteries in the body, especially those that supply blood to the head and neck. Symptoms may include headache, fatigue, and jaw pain. Effects can include stroke and blindness.
RoActemra can reduce the pain and swelling of the arteries and veins in the head, neck, and arms.
GCA is often treated with medications called steroids. They are usually effective, but can have side effects if used at high doses for a long time. Reducing the dose of steroids can also lead to a flare-up of GCA. The addition of RoActemra to treatment can make the duration of steroid use shorter, while still controlling GCA.
- children and adolescents, 1 year of age and older with active systemic juvenile idiopathic arthritis (sJIA), an inflammatory disease that causes pain and swelling in one or more joints, as well as fever and skin rash.
RoActemra is used to improve the symptoms of sJIA. It can be administered in combination with methotrexate or alone.
- children and adolescents, 2 years of age and older, with active polyarticular juvenile idiopathic arthritis (pJIA). This is an inflammatory disease that causes pain and swelling in one or more joints.
RoActemra is used to improve the symptoms of pJIA. It can be administered in combination with methotrexate or alone.
2. What you need to know before you use RoActemra
You will not be given RoActemra
- If you or a child (if they are the patient you are caring for) are allergic to tocilizumab or any of the other ingredients of this medicine (listed in section 6).
- If you or a child (if they are the patient you are caring for) have a severe active infection.
If any of these apply to you, talk to your doctor. Do not use RoActemra.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start receiving RoActemra.
- If you experience allergic reactionssuch as chest tightness, wheezing, dizziness, or severe dizziness, swelling of the lips, tongue, face, or skin rash, hives, or itching during or after the injection, tell your doctor immediately.
- If you have experienced any symptoms of an allergic reaction after administration of RoActemra, do not take the next dose until you have informed your doctor and your doctor has indicated that you can take the next dose.
- If you have any type of infection, either short-term or long-term, or if you get infections often. Tell your doctor immediatelyif you feel unwell. RoActemra may reduce the ability of your body to respond to infections and may make an existing infection worse or increase the likelihood of getting a new infection.
- If you have had tuberculosis, tell your doctor. Your doctor will check for signs and symptoms of tuberculosis before starting treatment with RoActemra. Tell your doctor immediately if symptoms of tuberculosis (persistent cough, weight loss, general malaise, low-grade fever), or any other infection appear during or after treatment.
- If you have had intestinal ulcer or diverticulitis, tell your doctor. Symptoms would include abdominal pain and unexplained changes in bowel habits with fever.
- If you have liver disease, tell your doctor. Before using RoActemra, your doctor will perform a blood test to measure your liver function.
- If a patient has been recently vaccinated, or is scheduled to be vaccinated, tell your doctor. All patients must be up to date with their vaccination schedule before starting treatment with RoActemra. Certain types of vaccines should not be administered while receiving RoActemra.
- If you have cancer, tell your doctor. Your doctor will have to decide if you can continue receiving treatment with RoActemra.
- If you have cardiovascular risk factors, such as high blood pressure, and high cholesterol levels, tell your doctor. These factors need to be controlled while receiving treatment with RoActemra.
- If you have moderate to severe kidney problems, your doctor will monitor you.
- If you have persistent headaches.
Your doctor will perform blood tests before you receive RoActemra to determine if you have a low white blood cell count, a low platelet count, or elevated liver enzymes.
Children and adolescents
RoActemra subcutaneous injection is not recommended in children under 1 year of age.
RoActemra should not be administered to children with sJIA with a weight below 10 kg.
If a child has a history of macrophage activation syndrome(uncontrolled activation and proliferation of specific blood cells), tell your doctor. Your doctor will have to decide if RoActemra can still be administered.
Other medicines and RoActemra
Tell your doctor if you are taking any other medicines, or have taken any recently. This is because RoActemra may affect the way some medicines work, and a dose adjustment may be needed. Tell your doctorif you have recently used medicines containing any of the following active substances:
- methylprednisolone, dexamethasone, used to reduce inflammation
- simvastatin or atorvastatin, used to reduce cholesterol levels
- calcium channel blockers, such as amlodipine, used in the treatment of high blood pressure
- theophylline, used in the treatment of asthma
- warfarin or phenprocoumon, used as anticoagulants
- phenytoin, used in the treatment of seizures
- cyclosporin, used in organ transplants as an immunosuppressant
- benzodiazepines, such as temazepam, used to calm anxiety.
Because there is no clinical experience, the use of RoActemra with other biologic medicines used to treat RA, sJIA, pJIA, or GCA is not recommended.
Pregnancy, breastfeeding, and fertility
RoActemra should not be used during pregnancy, unless clearly necessary. Talk to your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
Women of childbearing agemust use effective contraceptive methods during and up to 3 months after finishing treatment.
Stop breastfeeding if you start treatment with RoActemra, and consult your doctor. Before restarting breastfeeding, at least 3 months must have passed since your last treatment with RoActemra. It is not known if RoActemra passes into breast milk.
Driving and using machines
This medicine may cause dizziness, if you feel dizzy, do not drive or use machines.
RoActemra contains polysorbate
This medicine contains 0.18 mg of polysorbate 80 in each pre-filled syringe with 162 mg/0.9 ml, which is equivalent to 0.2 mg/ml. Polysorbates can cause allergic reactions. Tell your doctor if you or your child have any known allergies.
3. How to use RoActemra
Follow the instructions for administration of this medicine exactly as told by your doctor, pharmacist, or nurse. If you are unsure, talk to your doctor, pharmacist, or nurse.
Treatment should be started by a healthcare professional with experience in the diagnosis and treatment of RA, sJIA, pJIA, or GCA.
Recommended dose
The dose for adults with RA and GCA is 162 mg (the content of one pre-filled syringe) administered once a week.
Children and adolescents with sJIA (1 year of age and older)
The usual dose of RoActemra depends on the patient's weight.
- If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled syringe), once every 2 weeks
- If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled syringe), once a week.
Children and adolescents with pJIA (2 years of age and older)
The usual dose of RoActemra depends on the patient's weight.
- If the patient weighs less than 30 kg: the dose is 162 mg (the content of 1 pre-filled syringe), once every 3 weeks
- If the patient weighs 30 kg or more: the dose is 162 mg (the content of 1 pre-filled syringe), once every 2 weeks.
RoActemra is administered by injection under the skin (subcutaneously). At the start, your doctor or nurse may inject RoActemra for you. However, your doctor may decide that you can inject RoActemra yourself. In this case, you will receive information on how to self-inject RoActemra. Parents and caregivers will receive instructions on how to inject RoActemra to patients who cannot inject themselves, such as children.
Talk to your doctor if you have any questions about how to self-administer an injection or to the child you are caring for. At the end of this leaflet, you will find detailed "instructions for administration".
If you are given more RoActemra than you should
Because RoActemra is administered in a pre-filled syringe, it is unlikely that you will be given too much. However, if you are concerned, talk to your doctor, pharmacist, or nurse.
If an adult with RA and GCA or a child or adolescent with sJIA misses or forgets a doseIt is very important to use RoActemra exactly as your doctor prescribes. Keep a record of your next dose.
- If you miss your weekly dose within 7 days, take your dose on the next scheduled day.
- If you miss your every-other-week dose within 7 days, inject a dose as soon as you remember and administer your next dose according to your original schedule.
- If you miss your dose for 7 days or more, or are unsure when to inject RoActemra, call your doctor or pharmacist.
If a child or adolescent with pJIA misses or forgets a dose
It is very important to use RoActemra exactly as your doctor prescribes. Keep a record of your next dose.
- If you miss a dose within 7 days, inject a dose as soon as you remember and administer your next dose according to your original schedule.
- If you miss a dose for 7 days or more, or are unsure when to inject RoActemra, call your doctor or pharmacist.
If you stop treatment with RoActemra
You should not stop treatment with RoActemra without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects can occur up to at least 3 months after your last dose of RoActemra.
Serious side effects: talk to your doctor immediately.
Talk to your doctor immediatelyif you experience any of the following side effects:
These are common:May affect up to 1 in 10 people
Allergic reactionsduring or after the injection:
- difficulty breathing, chest tightness, or dizziness
- skin rash, itching, hives, swelling of the lips, tongue, or face
Signs of serious infections:
- fever and chills
- mouth or skin blisters
- stomach pain
Signs and symptoms of liver damage
May affect up to 1 in 1,000 people
- fatigue
- abdominal pain
- jaundice (yellowing of the skin or eyes)
List of other possible side effects
If you notice any of these symptoms, tell your doctor as soon as possible.
Very common side effects:
May affect more than 1 in 10 people
- upper respiratory tract infections, with typical symptoms such as cough, nasal congestion, runny nose, sore throat, and headache
- high levels of fat in the blood (cholesterol)
- reactions at the injection site
Common side effects:
May affect up to 1 in 10 people
lung infection (pneumonia)
- shingles (herpes zoster)
- fever (oral herpes), blisters
- skin infections (cellulitis), sometimes with fever and chills
- skin rash and itching, hives
- allergic reactions (hypersensitivity)
- eye infection (conjunctivitis)
- headache, dizziness, high blood pressure
- mouth ulcers, stomach pain
- fluid retention (edema) in the lower legs, weight gain
- cough, shortness of breath
- low white blood cell count in blood tests (neutropenia, leucopenia)
- abnormal liver function tests (elevated transaminases)
- increased bilirubin measured by blood tests
- low levels of fibrinogen in the blood (protein involved in blood clotting)
Uncommon side effects:
May affect up to 1 in 100 people
- diverticulitis (fever, nausea, diarrhea, constipation, stomach pain)
- swollen and red areas in the mouth
- high levels of fat in the blood (triglycerides)
- stomach ulcers
- kidney stones
- hypothyroidism
Rare side effects:
May affect up to 1 in 1,000 people
- Stevens-Johnson syndrome (skin rash that can lead to severe skin peeling)
- fatal allergic reactions (anaphylaxis)
- liver inflammation (hepatitis), jaundice
Very rare side effects:
May affect up to 1 in 10,000 people
- low white blood cell, red blood cell, and platelet count in blood tests
- liver failure
Side effects in children and adolescents with sJIA or pJIA
In children and adolescents with sJIA or pJIA, side effects are generally similar to those in adults. Some side effects are seen more frequently in children and adolescents: nasal and throat inflammation, headache, nausea, and decreased white blood cell count.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet.
You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of RoActemra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled syringe and on the carton after EXP. The expiry date refers to the last day of the month.
Store in a refrigerator (between 2 °C – 8 °C). Do not freeze. Once removed from the refrigerator, the pre-filled syringe can be stored for up to 2 weeks at a temperature not above 30 °C.
Keep the pre-filled syringes in the outer packaging to protect from light and moisture.
Do not use this medicine if you notice that it is cloudy or contains particles, is of a color other than colorless to slightly yellowish, or if any part of the pre-filled syringe appears to be damaged.
Do not shake the syringe. After removing the needle cap, the injection should be started within the next 5 minutes to avoid the medicine drying out and blocking the needle. If the pre-filled syringe is not used within 5 minutes of removing the needle cap, it should be disposed of in a puncture-resistant container and a new pre-filled syringe should be used.
If after inserting the needle you are unable to press the syringe plunger, the pre-filled syringe should be disposed of in a puncture-resistant container and a new pre-filled syringe should be used.
6. Package Contents and Additional Information
Composition of RoActemra
- The active substance is tocilizumab.
Each pre-filled syringe contains 162 mg of tocilizumab in 0.9 ml.
- The other ingredients are L-Histidine, L-Histidine monohydrochloride monohydrate, L-Arginine/L-Arginine hydrochloride, L-Methionine, Polysorbate 80, Water for injections (see section 2 “RoActemra contains polysorbate”).
Appearance and Package Contents of the Product
RoActemra is a solution for injection. The solution is colorless to slightly yellowish.
RoActemra is supplied in 0.9 ml pre-filled syringes containing 162 mg of tocilizumab solution for injection.
Each pack contains 4 pre-filled syringes. The multipack contains 12 (3 packs of 4) pre-filled syringes. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG Emil-Barell-Str. 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medicine from the local representative of the Marketing Authorisation Holder:
Belgium/Belgique/Belgien N.V. Roche S.A. Tel: +32 (0) 2 525 82 11 | Lithuania UAB “Roche Lietuva” Tel: +370 5 2546799 |
| Luxembourg/Luxemburg (See Belgium/Belgien) |
Czech Republic Roche s. r. o. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 - 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o Tel: +385 1 4722 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovak Republic Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency web site http://www.ema.europa.eu/.
What do I need to know to use my RoActemra pre-filled syringe safely?
It is important to read, understand, and follow the instructions so that you or your caregiver can use the RoActemra syringe correctly. These instructions do not replace the information that your healthcare professional should give you. Your healthcare professional will show you how to prepare and inject RoActemra properly before you use the RoActemra syringe for the first time. Ask your healthcare professional any questions you may have. Do not attempt to administer an injection until you are sure you understand how to use the RoActemra syringe.
Please also read the patient leaflet that comes with the RoActemra syringe for the most important information you need to know about this medicine. It is important that you remain under the care of your healthcare professional while being treated with RoActemra.
Important Information:
? Do not use it if the syringe appears to be damaged.
? Do not use it if the medicine is cloudy, hazy, discolored, or contains particles.
? Do not attempt to disassemble the syringe at any time.
? Do not remove the needle cap until you are ready for injection.
? Do not inject through clothing that covers your skin.
? Never reuse the same syringe.
? Do not touch the syringe's activation clips as this may damage the syringe.
Storage
Keep the RoActemra syringe and all medicines out of the sight and reach of children. Always store the syringe in the refrigerator at a temperature of 2-8 °C. Once removed from the refrigerator, the pre-filled syringe can be stored for up to a total of 2 weeks at a temperature not above 30 °C, but not beyond the original expiry date (EXP). Mark the date on the carton. The pre-filled syringe should always be stored in its original packaging. Protect the syringe from freezing and light. Keep the syringes dry.
Parts of the Pre-filled Syringe
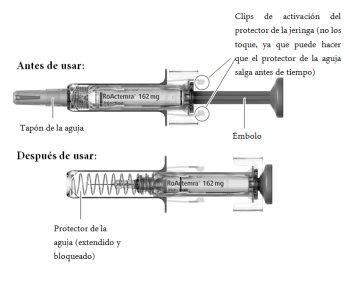
To Administer Your Injection, You Will Need:
Included in the pack:
? Pre-filled syringe.
Not included in the pack:
? Alcohol swab.
? Cotton or sterile gauze.
? Puncture-resistant container for safe disposal of the needle cap and used syringe.
A place to prepare your equipment:
? Find a well-lit, clean, and flat surface such as a table.
Step 1. Visually Check the Syringe
? Take the carton containing the RoActemra syringe from the refrigerator and open the carton. Do not touch the activation clips of the syringe's protector as this may damage the syringe.
? Remove the syringe from the carton and visually examine the syringe and the medicine in the syringe. This is important to ensure that the syringe and the medicine can be used safely.
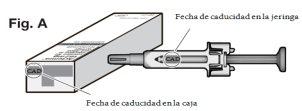
? Check the expiry date on the carton and on the syringe (see Fig. A) to ensure that it has not passed. Do not use the syringe if the expiry date has passed. This is important to ensure that the syringe and the medicine can be used safely.
Discard the Syringe and Do Not Use It If:
? The medicine is cloudy.
? The medicine contains particles.
? The medicine is of a color other than colorless to slightly yellowish.
? Any part of the syringe appears to be damaged.
Step 2. Allow the Pre-filled Syringe to Reach Room Temperature
? Do not remove the needle cap from your syringe before Step 5. Doing so may cause the medication to dry out and block the needle.
? Place the syringe on a clean, flat surface and allow the syringe to reach room temperature (18 °C – 28 °C) for 25-30 minutes to temper. If you do not allow the syringe to reach room temperature, it may result in an uncomfortable injection and may make it difficult to press the plunger.
? Do not heat the syringe in any other way.
Step 3. Wash Your Hands
- Wash your hands with soap and water.
Step 4. Choose and Prepare the Injection Site
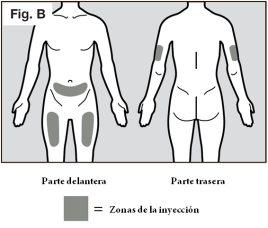
- Recommended injection sites are the front and middle of the thighs and the lower abdomen below the navel, except for the area of five centimeters immediately surrounding the navel (see Fig. B).
- If a caregiver is administering the injection, the outer aspect of the upper arms can also be used (see Fig. B).
? You should use a different site each time you administer an injection, at least 3 centimeters away from the site you used for your previous injection.
? Do not inject into areas where it may be rubbed by a belt or waistband. Do not inject into moles, scars, bruises, or areas where the skin is sensitive, red, hard, or broken.
? Clean the site using an alcohol swab (see Fig. C) to reduce the risk of infection.
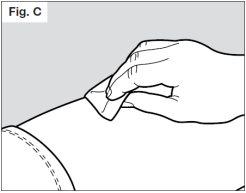
? Allow the skin to dry for about 10 seconds.
? Make sure you do not touch the cleaned area before the injection. Do not fan or blow on the cleaned area.
Step 5. Remove the Needle Cap
? Do not hold the syringe by the plunger while removing the needle cap.
? Hold the syringe's protector firmly with one hand and remove the needle cap with the other hand (see Fig. D). If you are unable to remove the needle cap, you should ask for help from your caregiver or contact your healthcare professional.
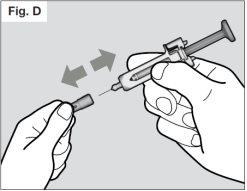
? Do not touch the needle or allow it to touch any surface.
- There may be a small air bubble in the RoActemra pre-filled syringe. It is not necessary to remove it.
? You may see a drop of liquid at the end of the needle. This is normal.
? Dispose of the needle cap in a puncture-resistant container.
NOTE: Once the needle cap is removed, the syringe should be used immediately.
? If the needle cap is removed and the syringe is not used within 5 minutes, the syringe should be disposed of in a puncture-resistant container and a new pre-filled syringe should be used. If the needle cap is removed and the syringe is not used within 5 minutes, it may be more difficult to administer the injection as the medicine may dry out and block the needle.
? Never replace the needle cap after it has been removed.
Step 6. Administer the Injection
? Hold the syringe comfortably in your hand.
? To ensure that the needle can be inserted correctly into your skin, pinch a fold of loose skin at the cleaned injection site with your free hand. Pinching the skin is important to ensure that you inject under your skin (into the fatty tissue) but not too deeply (into the muscle). Injection into the muscle could result in an uncomfortable injection.
? Do not hold or push the plunger while inserting the needle into the skin.
? Insert the needle completely into the fold of skin at an angle of 45° to 90° with a quick and firm motion (see Fig. E).
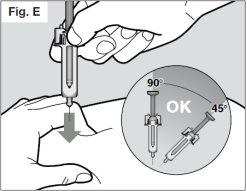
It is important to choose the correct angle to ensure that the medicine is released under the skin (into the fatty tissue), otherwise the injection could be painful and the medicine may not work.
? Afterward, hold the syringe in place and release the fold of skin.
? Inject slowly all of the medicine by pushing the plunger slowly until it reaches the end (see Fig. F). You must press the plunger all the way to ensure that the full dose of medicine is administered and to ensure that the syringe's activation clips are fully pushed to the side. If the plunger is not pressed all the way, the needle protector will not extend to cover the needle when it is removed. If the needle is not covered, proceed with caution and place the syringe in a puncture-resistant container to avoid damage from the needle.
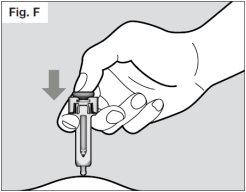
? Once the plunger is pressed all the way, hold it in place to ensure that all of the medicine is injected before removing the needle from the skin.
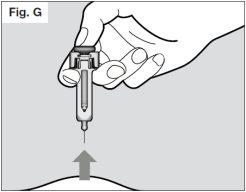
? Hold the plunger in place while pulling the needle out of the skin at the same angle that it was inserted (see Fig. G).
? If after inserting the needle you are unable to press the plunger, the pre-filled syringe should be disposed of in a puncture-resistant container and a new pre-filled syringe should be used (starting again from Step 2). If you still have difficulty, you should consult your healthcare professional.
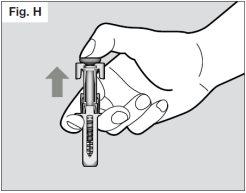
? Once the needle is completely removed from the skin, you can release the plunger, allowing the needle protector to protect the needle (see Fig. H).
? If you see drops of blood at the injection site, you can press the injection site with a sterile cotton ball or gauze for about 10 seconds.
? Do not rub the injection site.
Step 7. Disposal of the Syringe
? Do not attempt to recap your syringe.
? Dispose of used syringes in a puncture-resistant container. Ask your doctor or pharmacist for information on where you can get a puncture-resistant container or what other type of puncture-resistant container you can use for safe disposal of your used syringes if you do not have one (see Fig. I).
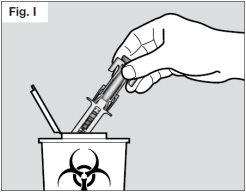
Talk to your healthcare professional for instructions on the best way to dispose of used syringes. There may be national regulations on how to dispose of used syringes.
Do not throw away used syringes or the puncture-resistant container in your household trash and do not recycle them.
? Dispose of the filled container as instructed by your doctor or pharmacist.
? Always keep the puncture-resistant container out of the sight and reach of children.
Patient Advice Related to Hypersensitivity Reactions (also known as Anaphylaxis, if severe)
If you develop symptoms such as, but not limited to, rash, itching, chills, swelling of the face, lips, tongue, or throat, chest pain, wheezing, difficulty breathing or swallowing, or feeling dizzy or faint at any time outside of the clinic, during or after the injection of RoActemra, you should seek immediate medical attention.
Patient Advice Related to Early Recognition and Treatment to Limit the Risk of Serious Infections
Be alert to the first signs of infection such as:
? Body aches, fever, chills.
? Cough, discomfort/pain or pressure in the chest, shortness of breath.
? Redness, warmth, unusual swelling of the skin or joints.
? Abdominal pain/tenderness and/or changes in bowel function.
Call your doctor and seek medical attention without delay if you think you may be developing an infection.
If you have any concerns or questions about your syringe, contact your doctor or pharmacist for help.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROACTEMRA 162 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 162 mgActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE, 162 mgActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: tocilizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription required
Online doctors for ROACTEMRA 162 mg Injectable Solution in Pre-filled Syringe
Discuss questions about ROACTEMRA 162 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







