
RIZATRIPTAN SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS

How to use RIZATRIPTAN SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rizatriptan Sandoz 10 mg Oral Lyophilisates EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Rizatriptan Sandoz and what is it used for
- What you need to know before you take Rizatriptan Sandoz
- How to take Rizatriptan Sandoz
- Possible side effects
- Storage of Rizatriptan Sandoz
- Contents of the pack and other information
1. What is Rizatriptan Sandoz and what is it used for
Rizatriptan Sandoz contains the active substance rizatriptan, which belongs to a group of medicines called selective serotonin 5-HT1B/1D receptor agonists.
Rizatriptan is used to treat migraine headaches in adults.
2. What you need to know before you take Rizatriptan Sandoz
Do not take Rizatriptan Sandoz if:
- you are allergic to rizatriptan or any of the other ingredients of this medicine (listed in section 6),
- you are currently taking monoamine oxidase inhibitors (MAOIs) such as moclobemide, phenelzine, tranylcypromine (used to treat depression) or linezolid (an antibiotic), or if you have taken an MAOI in the last two weeks (see section “Taking Rizatriptan Sandoz with other medicines”),
- you have severely impaired kidney or liver function,
- you have had a stroke or symptoms similar to a stroke that lasted for a few days (transient ischaemic attack, TIA),
- you have high blood pressure that is not controlled by medication,
- you have had heart disease (poor circulation of blood in the coronary arteries), a heart attack, or a certain type of chest pain known as Prinzmetal's angina,
- you have had circulation problems in your legs (peripheral vascular disease),
- you are taking another migraine medicine which contains ergotamine, or other medicines of the same type (e.g. dihydroergotamine, methysergide) or other 5-HT1B/1D receptor agonists (e.g. sumatriptan, naratriptan, zolmitriptan) (see section “Taking Rizatriptan Sandoz with other medicines”).
Warnings and precautions
Consult your doctor or pharmacist before taking Rizatriptan Sandoz if you:
- have any of the following risk factors for heart disease:
- high blood pressure or diabetes,
- are a smoker or use nicotine replacement therapy,
- have a family history of heart disease,
- are a man over 40 years of age or a postmenopausal woman,
- have kidney or liver problems,
- have a specific heart rhythm disorder (bundle branch block),
- have had any allergies,
- have had headache associated with dizziness, difficulty walking, lack of coordination or weakness in the legs and arms,
- have had allergic reactions such as swelling of the face, lips, tongue and/or throat which may cause difficulty breathing or swallowing, caused by this medicine or other medicines of the same type (angioedema),
- have had transient symptoms including pain and pressure in the chest.
If you take rizatriptan too frequently, it may cause you to have chronic headaches. In such cases, you should contact your doctor immediately and stop taking these tablets.
Please inform your doctor of all your symptoms. Your doctor will decide if you have migraines. You should only take rizatriptan for a migraine attack.Rizatriptan should not be used to treat other types of headaches that may be caused by other, more serious conditions.
If you are over 65 years old, your doctor will advise you whether you can take these tablets.
Children and adolescents
Rizatriptan is not recommended for use in children under 18 years of age.
Other medicines and Rizatriptan Sandoz
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Do not take rizatriptan with:
monoamine oxidase inhibitors (MAOIs)such as moclobemide, phenelzine, linezolid or tranylcypromine, or if you have taken an MAOI in the last two weeks.
certain migraine medicines, e.g.
- other medicines of the same type as rizatriptan, such as sumatriptan, naratriptan or zolmitriptan,
- ergotamine-like medicines, such as ergotamine, dihydroergotamine or methysergide. After taking rizatriptan, you should wait at least 6 hours before taking these medicines, and wait at least 24 hours before starting to take ergotamine-like medicines after you have finished taking rizatriptan.
Ask your doctor for instructions on how to take rizatriptan and information about the risks if you are also taking:
- antidepressants such as sertraline, escitalopram, fluoxetine, venlafaxine and duloxetine,
- propranolol (usually used to treat high blood pressure) - you should only take the lower dose of 5 mg of rizatriptan,
- a herbal remedy called St. John's Wort (Hypericum perforatum). Taking it with rizatriptan may increase the chance of getting side effects. It is recommended that you do not take rizatriptan at the same time.
Taking Rizatriptan Sandoz with food and drink
It is best to take these tablets on an empty stomach, but you can take them with food if you prefer. If you take rizatriptan with food, it may take longer to work.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Available data on the use of rizatriptan in the first three months of pregnancy do not indicate an increased risk of birth defects.
It is not known whether rizatriptan is harmful when taken by pregnant women after the first three months of pregnancy.
If you are breast-feeding, you may breast-feed for up to 12 hours after treatment to avoid exposing your baby.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Migraine or treatment with rizatriptan may cause drowsiness in some patients. Dizziness has also been reported in some patients taking this medicine. If you experience these effects, you should make sure you are safe to drive or operate machinery.
Rizatriptan Sandoz contains aspartame, sodium and sulphites
This medicine contains 5.6 mg of aspartame in each orodispersible tablet. Aspartame is a source of phenylalanine, which may be harmful for people with phenylketonuria (a rare genetic disorder in which phenylalanine builds up because the body cannot remove it properly).
This medicine contains less than 23 mg of sodium (1 mmol) per orodispersible tablet, which is essentially “sodium-free”.
This medicine may cause severe allergic reactions and bronchospasm (a sudden feeling of tightness in the chest) because it contains sulphites.
3. How to take Rizatriptan Sandoz
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Rizatriptan is not used to prevent migraine. It only works when a migraine attack has started.
The recommended dose for adults over 18 yearsis 10 mg at the first sign of a migraine headache. However, for some patients the recommended dose is 5 mg. Your doctor will decide the right dose for you and it is important that you take the medicine as instructed by your doctor.
Most migraine attacks are relieved by a single dose (one tablet) of rizatriptan, but if you do not get relief from the headache after taking one tablet, do not take another tablet for the same attackand consult your doctor.
Even if a migraine attack does not respond to the first dose of rizatriptan, it is still likely that you will respond to rizatriptan during the next migraine attack.
If you get another migraine attack within 24 hours of taking the first tablet, you can take another dose of Rizatriptan Sandoz. However, do not take more than two doses in a 24-hour period. Always wait at least 2 hours between doses.
How to take the tablets
Take the orodispersible tablets as follows:
- Separate the blister along the perforation.
- Pull the blister tab carefully from the arrow, as shown in the picture.
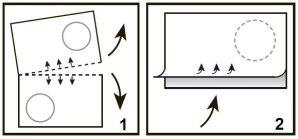
Place the tablet on your tongue, where it will dissolve and can be swallowed with saliva. You do not need to drink water to swallow the tablet.
If you take more Rizatriptan Sandoz than you should
It is important to use the dose prescribed by your doctor. If you have taken more tablets than your doctor prescribed, you should contact a doctor or pharmacist immediately. Taking too many tablets may harm you. The effects of taking too many tablets may include effects similar to those described in section 4, especially: dizziness, drowsiness, fainting and slowing of heart rate. You may also experience increased blood pressure and side effects that affect the heart and circulation.
If you have taken more Rizatriptan Sandoz than you should, contact your doctor or pharmacist immediately, or call the Toxicology Information Service on +34 91 562 04 20, stating the name and quantity of the medicine taken.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In adults, the most commonly reported side effects were dizziness, drowsiness and fatigue.
Tell your doctor immediately if you have symptoms of:
- allergic reactions, sometimes very severe, including swelling of the face, lips, tongue and throat which may cause difficulty breathing, speaking or swallowing (angioedema),
- severe allergic reaction with rash, red skin, blisters on the lips, eyes or mouth, skin peeling and fever,
- chest pain, tightness in the chest or throat, or other symptoms that may indicate a heart attack,
- weakness or paralysis of the limbs or face, difficulty speaking, which may indicate a stroke,
- a syndrome called “serotonin syndrome” characterized by coma, unstable blood pressure, extremely high body temperature, lack of muscle coordination, agitation and hallucinations.
Side effects
Common(may affect up to 1 in 10 people):
- dizziness, drowsiness and feeling weak/tired,
- headache, tingling sensation (paraesthesia), decreased sensitivity of the skin (hypoesthesia), decreased mental sharpness, insomnia,
- fast or irregular heart beat (palpitations),
- throat discomfort,
- nausea (feeling sick), dry mouth, vomiting, diarrhoea, indigestion,
- flushing (short-term redness of the face),
- feeling of heaviness in any part of the body, neck pain, stiffness,
- stomach or chest pain.
Uncommon(may affect up to 1 in 100 people):
- lack of muscle coordination, disorientation, nervousness, feeling of spinning,
- tremor,
- fainting,
- altered taste (bad taste),
- blurred vision,
- high blood pressure,
- hot flushes,
- difficulty breathing,
- thirst,
- rash, itching, rash and itching, hives, swelling of the face, lips, tongue and/or throat which may cause difficulty breathing and/or swallowing (angioedema),
- sweating,
- feeling of tightness in any part of the body, muscle weakness,
- irregular heart rhythm, abnormal heart tracing (ECG) (a test that records the electrical activity of the heart),
- facial pain, muscle pain.
Rare(may affect up to 1 in 1,000 people):
- wheezing,
- allergic reaction (hypersensitivity); sudden life-threatening allergic reaction (anaphylaxis),
- stroke (this usually occurs in patients with risk factors for heart and blood vessel disease [high blood pressure, diabetes, smoking, use of nicotine replacement therapy, family history of heart disease or stroke, men over 40 years of age, postmenopausal women, in particular heart rhythm disorders (bundle branch block)]),
- slow heart rate.
Frequency not known(cannot be estimated from the available data):
- spasm of the blood vessels in the arms and legs, including coldness and numbness of the hands or feet,
- seizures,
- a syndrome called “serotonin syndrome” which can cause reactions such as coma, unstable blood pressure, extremely high body temperature, lack of muscle coordination, agitation and hallucinations,
- severe skin peeling with or without fever (toxic epidermal necrolysis),
- ischaemic colitis (inflammation that causes abdominal pain or diarrhoea),
- heart attack or spasm of the blood vessels of the heart. These usually occur in patients with risk factors for heart and blood vessel disease. The risk factors are high blood pressure, diabetes, smoking, use of nicotine replacement therapy, family history of heart disease or stroke, men over 40 years of age, postmenopausal women, in particular heart rhythm disorders (bundle branch block).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rizatriptan Sandoz
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister and on the carton after EXP. The expiry date refers to the last day of that month.
Store in the original package to protect from moisture.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Packaging Content and Additional Information
Composition of Rizatriptan Sandoz
The active ingredient is rizatriptan. Each orodispersible tablet contains 10 mg of rizatriptan (as benzoate).
The other components are: calcium silicate, crospovidone type A, anhydrous colloidal silica, silicified microcrystalline cellulose, mannitol (E 421), aspartame (E 951), magnesium stearate, sweet orange flavor (contains gum arabic (E 414), ascorbic acid (E 300), ethyl butyrate, maltodextrin, orange oil, propylene glycol (E 1520), sodium, sulfites).
Product Appearance and Packaging Content
Orodispersible tablet.
White to grayish-white, round, flat tablets, marked with "RZT" on one side and "10" on the other.
The orodispersible tablets are packaged in aluminum/aluminum blisters, which are presented in a cardboard box.
Package sizes: 2, 3, 6, 12, 18 orodispersible tablets.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer
Lek Pharmaceuticals, d.d.
Verovškova 57
1526 Ljubljana
Slovenia
or
Lek Pharmaceuticals, d.d.
Trimlini 2D
9220 Lendava
Slovenia
or
LEK S.A.
Ul. Podlipie 16
95-010 Stryków
Poland
or
Lek, S.A.
Ul Domaniewska 50 C
PL02-672 Warsaw
Poland
or
SANDOZ
SRL Livezeni Street, 7A
RO-540472 Targu Mures
Romania
or
Salutas Pharma GmbH
Otto Von Guericke Alle, 1
D-39179 Barleben
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Denmark: Rizatriptan Sandoz
Germany: Rizatriptan lingual – 1 A Pharma 10 mg Schmelztabletten
Italy: RIZATRIPTAN SANDOZ 10 mg compresse orodispersibili
Netherlands: Rizatriptan Sandoz 10 mg, orodispergeerbare tabletten
Slovakia: Rizatriptan Sandoz 10 mg orodispergovatelné tablety
Spain: Rizatriptán Sandoz 10 mg comprimidos bucodispersables EFG
Sweden: Rizatriptan Sandoz 10 mg munsönderfallande tablett
United Kingdom: Rizatriptan 10 mg Orodispersible Tablets
Date of the last revision of this leaflet:July 2024.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price8.8 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RIZATRIPTAN SANDOZ 10 mg ORALLY DISINTEGRATING TABLETSDosage form: TABLET, 10 mgActive substance: rizatriptanManufacturer: Organon Salud S.L.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 10 mgActive substance: rizatriptanManufacturer: Organon Salud S.L.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 10 mg / tabletActive substance: rizatriptanManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for RIZATRIPTAN SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about RIZATRIPTAN SANDOZ 10 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















