
RHINOVIN 1 mg/ml NASAL SPRAY SOLUTION

How to use RHINOVIN 1 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PROSPECTUS: INFORMATION FOR THE USER
Rhinovín1 mg/ml nasal spray solution
Xylometazoline hydrochloride
Read this prospectus carefully because it contains important information for youThis medication can be purchased without a prescription. Nevertheless, to obtain the best results, it should be used properly.
- Keep this prospectus, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If the symptoms worsen or persist after 3 days, you should consult a doctor.
- If you consider that any of the adverse effects you are experiencing is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
Contents of the prospectus:
- What Rhinovín is and what it is used for
- Before using Rhinovín
- How to use Rhinovín
- Possible side effects
5 Conservation of Rhinovín
- Additional information
1. What is RHINOVÍN and what is it used for
This medication belongs to the group of medications called Sympathomimetics.
It is a nasal decongestant medication
It contains xylometazoline as the active ingredient. Xylometazoline, administered in the form of a nasal spray, produces a local constriction of blood vessels, decongesting the nasal mucosa.
Rhinovín is indicated for the local and temporary relief of nasal congestion.
2. BEFORE USING RHINOVÍN
Do not use Rhinovín:
- if you are allergic (hypersensitive) to xylometazoline, to other nasal decongestants, or to any of the other components (excipients) of the medication
- If you have recently undergone an operation on the head (if you have undergone any cranial, transnasal, or transoral surgical intervention).
Be careful with Rhinovín
Be careful with Rhinovín and consult your doctor before using this medication if:
- You are being treated with tricyclic or tetracyclic antidepressant medications, phenothiazine (a tranquilizer), or methyldopa (to lower blood pressure).
- You have had or have any of the following diseases or symptoms:
- If you have high blood sugar levels (diabetes mellitus)
- If you have high blood pressure (arterial hypertension)
- If you have any heart or circulatory disease (e.g., long QT syndrome);
- If you have any prostate disease with difficulty urinating (prostatic hypertrophy)
- If you have any thyroid disease (hyperthyroidism)
- If you have ever experienced insomnia or vertigo while being treated with other sympathomimetic medications, such as those used to treat heart diseases, hypotension (low blood pressure), or asthma.
- In rare cases, xylometazoline may increase nasal congestion instead of decreasing it due to its temporary effects and prolonged use; this is known as rebound effect.
- Rarely, insomnia may occur after using the medication. If this happens, avoid using it in the late afternoon or evening.
Do not exceed the recommended dose in section 3 HOW TO USE Rhinovín.
To avoid contagion, the medication should not be used by more than one person, and the applicator should be cleaned after each use with a clean, damp cloth.
Use of other medications
Inform your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
This medication should not be used by people who are taking or have taken during the last two weeks: medications used to treat depression (tricyclic or tetracyclic antidepressants or monoamine oxidase inhibitors (MAOIs)), or a medication to lower blood pressure called methyldopa.
It should also not be used in case of treatment with phenothiazine (a tranquilizer) or with medications to treat asthma.
Use in children:
Rhinovín should not be used in children under 6 years old without the recommendation of a doctor. Children may be more prone to the adverse effects of this medication.
Use in people over 65 years old:
Consult your doctor or pharmacist, as older people are more sensitive to the effects of this medication.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
This medication should not be used during pregnancy or breastfeeding.
Driving and using machines
Although problems are not expected, if you experience drowsiness or dizziness while using this medication, do not drive or operate hazardous tools or machines.
Important information about some of the components of Rhinovín:
This medication may cause inflammation of the nasal mucosa, especially with long-term treatments, because it contains benzalkonium chloride. If such a reaction is suspected (persistent nasal congestion), whenever possible, a nasal medication that does not contain this excipient should be used.
3. HOW TO USE RHINOVÍN
Follow these instructions unless your doctor has given you different instructions. Consult your doctor or pharmacist if you have doubts.
Adults and children from 12 years old:
The normal dose is: 1 spray in each nostril per application. 1 application per day, if necessary, a second application can be repeated (every 10 to 12 hours). Do not exceed 2 applications in 24 hours.
This medication is used nasally.
The dosing spray ensures good distribution of the solution over the surface of the nasal mucosa through a fine nebulization.
Before using for the first time
Pump the spray 5 times. Once primed, the pump will remain charged normally throughout the daily treatment period.
If the spray does not expel during complete actuation, or if the medication has not been used for more than 7 days, it will be necessary to re-prime the pump with 2 actuations.
If the full spray is not administered, the dose should not be repeated.
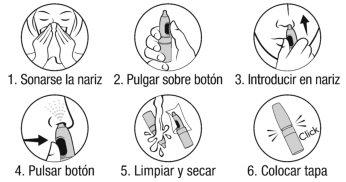
Instructions for use:
Remove the cap.
Be careful not to spray into the eyes.
- Blow your nose.
- Hold the bottle vertically with your thumb on the actuation button.
- To avoid dripping, stay upright and insert the spray nozzle into one nostril.
- Press the button to spray and inhale gently through the nose at the same time.
Repeat this process (steps 2 to 4) in the other nostril.
- After each use, clean and dry the nozzle.
- Put the protective cap back on until you hear a "click".
To avoid possible spread of infection, the spray should only be used by one person.
If the symptoms worsen or persist after 3 days of treatment, discontinue treatment and consult your doctor.
If you use more Rhinovín than you should:
Due to excessive or very continuous dosing, you may notice: headache, tremors, insomnia, excessive sweating, palpitations, tachycardia, increased blood pressure, or sleep disturbances.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Rhinovín:
Do not use a double dose to make up for a forgotten dose.
If necessary, use it again as indicated in section 3 HOW TO USE Rhinovín
4. Possible side effects
Like all medications, Rhinovín can cause side effects, although not everyone experiences them.
During the use of xylometazoline, the following side effects have been observed, whose frequency has not been established with precision:
The most frequent side effects are:
Irritation at the site of application, dryness, itching of the nasal mucosa, or sneezing.
The side effects that have occurred with low frequency are:
Nasal bleeding
The side effects that can occur rarely are:
Anxiety, restlessness, insomnia, hallucinations, tremors, and sleep disorders in children. Tachycardia, palpitations, increased blood pressure. Headache, nausea, exanthema (skin redness).
Like other nasal decongestants, excessive or continuous use of Rhinovín can lead to nasal congestion due to rebound effect.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this prospectus, inform your doctor or pharmacist.
5. Conservation of RHINOVÍN
Keep out of the reach and sight of children.
Store below 30°C.
Do not use Rhinovín after the expiration date shown on the packaging. The expiration date is the last day of the month indicated. This date applies even if the packaging has been opened.
Medications should not be thrown away through the drains or into the trash. Ask your pharmacist how to dispose of the packaging and medications that are no longer needed. This will help protect the environment.
6. ADDITIONAL INFORMATION
Composition of Rhinovín
- The active ingredient is xylometazoline hydrochloride. Each milliliter of this solution contains 1mg of xylometazoline, and each spray contains 138 micrograms of xylometazoline hydrochloride.
- The other components (excipients) are sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, disodium edetate, benzalkonium chloride, sorbitol (E420), hypromellose, sodium chloride, and purified water.
Appearance of the product and packaging content
Rhinovín is a solution presented in a nasal spray with 10 ml.
Marketing authorization holder
Haleon Spain, S.A. Paseo de la Castellana, 259D, 32nd floor - 28046 – Madrid – Spain.
Manufacturer
Novartis Consumer Health GmbH
Zielstattstrasse 40
81379 Munich
Germany
Haleon Germany GmbH
Barthstraße 4
80339 Munich
Germany
This prospectus was approved in January 2024.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RHINOVIN 1 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 1 mgActive substance: xylometazolineManufacturer: Laboratorio De Aplicaciones Farmacodinamicas S.A.Prescription not requiredDosage form: NASAL PRODUCT, Xylometazoline hydrochloride 0.1%Active substance: xylometazolineManufacturer: Kern Pharma S.L.Prescription not requiredDosage form: NASAL PRODUCT, 0.5 mgActive substance: xylometazolineManufacturer: Haleon Spain S.A.Prescription not required
Online doctors for RHINOVIN 1 mg/ml NASAL SPRAY SOLUTION
Discuss questions about RHINOVIN 1 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











