RESPREEZA 5,000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION

How to use RESPREEZA 5,000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Respreeza 1,000 mg powder and solvent for solution for infusion
Respreeza 4,000 mg powder and solvent for solution for infusion
Respreeza 5,000 mg powder and solvent for solution for infusion
Human alpha1-proteinase inhibitor
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Respreeza and what is it used for
- What you need to know before you use Respreeza
- How to use Respreeza
- Possible side effects
- Storage of Respreeza
- Contents of the pack and other information
1. What is Respreeza and what is it used for
What is Respreeza
This medicine contains the active substance human alpha1-proteinase inhibitor, which is a normal component of blood and is found in the lungs, where its main function is to protect lung tissue by limiting the action of a certain enzyme called neutrophil elastase. This enzyme can cause damage if its action is not controlled (for example, if you have a deficiency of alpha1-proteinase inhibitor).
What Respreeza is used for
This medicine is used in adults with a known severe deficiency of alpha1-proteinase inhibitor (a hereditary condition also known as alpha1-antitrypsin deficiency) who have developed a lung condition called emphysema.
Emphysema develops when the lack of alpha1-proteinase inhibitor affects the proper control of neutrophil elastase, damaging the small air sacs in the lungs through which oxygen passes into the body. As a result of this damage, the lungs do not function properly.
Regular use of this medicine increases blood and lung concentrations of alpha1-proteinase inhibitor, thereby slowing the progression of emphysema.
2. What you need to know before you use Respreeza
Do not use Respreeza
- if you are allergic to human alpha1-proteinase inhibitor or any of the other ingredients of this medicine (listed in section 6).
- if you have been determined to have a deficiency of certain blood proteins called immunoglobulin A (IgA) and have developed antibodies against them.
Warnings and precautions
- Talk to your doctor or healthcare professional before using Respreeza.
Information on allergic reactions: when should you stop or slow down the infusion?
You may be allergic to human alpha1-proteinase inhibitor even if you have previously received human alpha1-proteinase inhibitors and tolerated them well. In some cases, severe allergic reactions can occur. Your doctor will inform you about the signs of allergic reactions (such as chills, flushing, faster heartbeat, drop in blood pressure, dizziness, rash, hives, itching, difficulty breathing or swallowing, as well as swelling of your hands, face, or mouth) (see also section 4).
- If you notice such reactions during the infusion of your medicine, inform your doctor or healthcare professional immediately. Depending on the type and intensity of the reaction, your doctor may decide to slow down or stop the infusion and initiate appropriate treatment.
- In case of self-administration/home treatment, stop the infusion immediatelyand contact your doctor or healthcare professional.
Information on safety with respect to infections
Respreeza is made from human blood plasma (the liquid part of the blood from which the blood cells have been removed).
Because infections can be transmitted through blood, when medicines are made from blood or human plasma, certain measures are taken to prevent the presence of infections in the medicine and their transmission to patients. These measures include:
- careful selection of blood and plasma donors to ensure the exclusion of individuals who may be carriers of infections,
- testing of donated blood and plasma samples to try to avoid the use of material with signs of viruses or infections,
- inclusion of measures in the processing of blood or plasma that allow the inactivation or removal of viruses.
These measures are considered effective for viruses such as human immunodeficiency virus (HIV), hepatitis A virus, hepatitis B virus, hepatitis C virus, and parvovirus B19.
However, despite these measures, when administering medicines prepared from human blood or plasma, it cannot be completely excluded that the possibility of transmitting an infection. This also applies to any unknown or emerging virus or other types of infections.
Your doctor may recommend that you consider vaccination against hepatitis A and B if you receive regular/repeated treatment with plasma-derived proteinase inhibitors.
Smoking
Because tobacco smoke is a major risk factor for the development and progression of emphysema, it is strongly recommended that you stop smoking and avoid passive exposure to tobacco smoke.
Children and adolescents
This medicine is not intended for use in children or adolescents under 18 years of age.
Using Respreeza with other medicines
- Tell your doctor or healthcare professional if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or healthcare professional for advice before using this medicine.
Since alpha1-proteinase inhibitor is a normal component of human blood, it is not expected that the recommended dose of this medicine will cause any harm to the developing fetus. However, because there is no information on the safety of using Respreeza during pregnancy, if you are pregnant, this medicine should only be used with caution.
It is not known whether Respreeza passes into breast milk. If you are breastfeeding, your doctor will explain the risks and benefits of using this medicine.
There are no data on the effects on fertility, although since alpha1-proteinase inhibitor is a normal component of blood, it is not expected to cause adverse effects on fertility if you use Respreeza at the recommended dose.
Driving and using machines
Dizziness may occur after administration of this medicine. If you feel dizzy, do not drive or use machines until the dizziness has passed (see section 4).
Respreeza contains sodium
Respreeza 1,000 mg powder and solvent for solution for infusion:
This medicine contains approximately 37 mg of sodium (the main component of cooking/table salt) in each vial of Respreeza 1,000 mg. This is equivalent to 1.9% of the maximum recommended daily intake of sodium for an adult.
Respreeza 4,000 mg powder and solvent for solution for infusion:
This medicine contains approximately 149 mg of sodium (the main component of cooking/table salt) in each vial of Respreeza 4,000 mg. This is equivalent to 7.4% of the maximum recommended daily intake of sodium for an adult.
Respreeza 5,000 mg powder and solvent for solution for infusion:
This medicine contains approximately 186 mg of sodium (the main component of cooking/table salt) in each vial of Respreeza 5,000 mg. This is equivalent to 9.3% of the maximum recommended daily intake of sodium for an adult.
Your doctor or healthcare professional will take this into account if you are on a controlled sodium diet.
3. How to use Respreeza
After reconstitution, Respreeza is administered by infusion into a vein. A healthcare professional with experience in the treatment of alpha1-proteinase inhibitor deficiency will supervise the first infusions.
Home treatment/Self-administration
After the first infusions, you or a person responsible for your care may also administer Respreeza, but only after receiving adequate training. If your doctor decides that you are suitable for home treatment/self-administration, they will teach you about:
- how to prepare and administer this medicine (see the illustrated instructions at the end of this leaflet in "Information for healthcare professionals and for patients suitable for home treatment/self-administration")
- how to keep the product sterile (aseptic infusion techniques)
- how to keep a daily treatment record
- how to identify side effects, including signs of allergic reactions, and the measures to be taken if such effects occur (see also section 2 and section 4)
Your doctor or healthcare professional will regularly review your infusion technique or that of the person responsible for your care to ensure that it is still being performed correctly.
Dose
The amount of Respreeza administered to you is based on your body weight. The recommended dose is 60 mg per kg of body weight and should be administered once a week. The infusion solution is usually administered over about 15 minutes (approximately 0.08 ml of solution per kg of body weight per minute). Depending on your weight and tolerability to the infusion, your doctor will determine the appropriate infusion rate for you.
If you use more Respreeza than you should
The consequences of an overdose are not known.
- Tell your doctor or healthcare professional if you think you have used more Respreeza than you should, so that the necessary measures can be taken.
If you forget to use Respreeza
- Apply the next dose immediately and continue at regular intervals, following the instructions of your doctor or healthcare professional.
- Do not take a double dose to make up for the forgotten dose.
If you stop using Respreeza
- Do not stop using this medicine without talking to your doctor or healthcare professional first. If you stop using Respreeza, your condition may worsen.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. These adverse reactions can occur even if you have previously received human alpha1-proteinase inhibitors and tolerated them well.
Some side effects can be serious:
Rare allergic reactions (may affect up to 1 in 100 people) have been observed.
In some very rare cases (may affect up to 1 in 10,000 people), they can become severe allergic reactions, even if you have not shown signs of allergy with previous infusions.
- Tell your doctor or healthcare professional immediatelyif you notice any signs of allergic reactions (such as chills, flushing, faster heartbeat, drop in blood pressure, dizziness, rash, hives, itching, difficulty breathing or swallowing, as well as swelling of your hands, face, or mouth) during the administration of Respreeza.
Depending on the type and intensity of the reaction, your doctor or healthcare professional may decide to slow down or stop the infusion and initiate appropriate treatment.
In case of self-administration/home treatment, stop the infusion immediatelyand contact your doctor or healthcare professional.
Other side effectsmay include:
Common(may affect up to 1 in 10 people)
Dizziness, headache, difficulty breathing (dyspnea), nausea.
Uncommon(may affect up to 1 in 100 people)
Altered sensation of touch such as burning, tingling, or numbness in your hands, arms, legs, or feet (paresthesia), flushing, rash (urticaria), scaly rash, and rash all over the body, physical weakness (asthenia), reactions at the infusion site (such as burning, stinging, pain, swelling, or redness at the infusion site (hematoma)).
Rare(may affect up to 1 in 10,000 people)
Decreased sensation of touch such as burning, tingling, or numbness in your hands, arms, legs, or feet (hypoesthesia), excessive sweating (hyperhidrosis), itching, chest pain, chills, fever (pyrexia).
Frequency not known(cannot be estimated from the available data)
Pain in lymph nodes (oval-shaped tissue masses located throughout the body that can be felt, for example, in the armpits, groin, or neck), swelling of the face, eyes, and lips.
Reporting of side effects
- If you experience any side effects, talk to your doctor or healthcare professional, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Respreeza
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the labels of the vials after EXP. The expiry date is the last day of the month stated.
Do not store above 25 °C. Do not freeze.
After reconstitution, the solution should be used immediately. If this is not possible, the solutions can be stored for up to 3 hours at room temperature (up to 25 °C). Do not freeze the reconstituted solution.
6. Container Contents and Additional Information
Composition of Respreeza
The active ingredientis human alpha1 proteinase inhibitor. One vial contains approximately 1,000 mg, 4,000 mg, or 5,000 mg of human alpha1 proteinase inhibitor.
The other componentsare sodium chloride, sodium dihydrogen phosphate monohydrate, and mannitol (see section 2).
Solvent: water for injectable preparations.
Appearance of the Product and Container Contents
This medicinal product is a white to off-white powder.
After reconstitution with water for injectable preparations, the solution should be clear, colorless to slightly yellowish, and free from visible particles.
Presentation:
Contents of one container:
Respreeza 1,000 mg powder and solvent for solution for infusion
- 1 single-use vial with powder
- 1 vial of solvent with 20 ml of water for injectable preparations
- 1 Mix2Vial 20/20 transfer device for reconstitution
Respreeza 4,000 mg powder and solvent for solution for infusion
- 1 single-use vial with powder
- 1 vial of solvent with 76 ml of water for injectable preparations
- 1 Mix2Vial 20/20 transfer device for reconstitution
Administration equipment (inner box):
- 1 IV infusion set
- 1 butterfly infusion catheter
- 3 alcohol-impregnated swabs
Respreeza 5,000 mg powder and solvent for solution for infusion
- 1 single-use vial with powder
- 1 vial of solvent with 95 ml of water for injectable preparations
- 1 Mix2Vial 20/20 transfer device for reconstitution
Administration equipment (inner box):
- 1 IV infusion set
- 1 butterfly infusion catheter
- 3 alcohol-impregnated swabs
Only certain pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
D-35041 Marburg
Germany
For further information about this medicinal product, please contact the local representative of the Marketing Authorisation Holder:
België/Belgique/Belgien CSL Behring NV Tél/Tel: +32 15 28 89 20 | Lietuva CentralPharma Communications UAB Tel: +370 5 243 0444 |
| Luxembourg/Luxemburg CSL Behring NV Tél/Tel: +32 15 28 89 20 |
Ceská republika CSL Behring s.r.o. Tel: +420 702 137 233 | Magyarország CSL Behring Kft. Tel.: +36 1 213 4290 |
Danmark CSL Behring AB Tlf: +46 8 544 966 70 | Malta AM Mangion Ltd. Tel: +356 2397 6333 |
Deutschland CSL Behring GmbH Tel: +49 6190 75 84810 | Nederland CSL Behring BV Tel: +31 85 111 96 00 |
Eesti CentralPharma Communications OÜ Tel: +3726015540 | Norge CSL Behring AB Tlf: +46 8 544 966 70 |
Ελλάδα CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Österreich CSL Behring GmbH Tel: +43 1 80101 1040 |
España CSL Behring S.A. Tel: +34 933 67 1870 | Polska CSL Behring Sp. z.o.o. Tel.: +48 22 213 22 65 |
France CSL Behring SA Tél: +33 1 53 58 54 00 | Portugal CSL Behring Lda Tel: +351 21 782 62 30 |
Hrvatska Marti Farm d.o.o. Tel: +385 1 5588297 | România Prisum Healthcare S.R.L. Tel: +40 21 322 01 71 |
Ireland CSL Behring GmbH Tel: +49 6190 75 84700 | Slovenija EMMES BIOPHARMA GLOBAL s.r.o.-podružnica v Sloveniji Tel:+ 386 41 42 0002 |
Ísland CSL Behring AB Sími: +46 8 544 966 70 | Slovenská republika CSL Behring Slovakia s.r.o. Tel: +421 911 653 862 |
Italia CSL Behring S.p.A. Tel: +39 02 34964 200 | Suomi/Finland CSL Behring AB Puh/Tel: +46 8 544 966 70 |
Κύπρος CSL Behring ΕΠΕ Τηλ: +30 210 7255 660 | Sverige CSL Behring AB Tel: +46 8 544 966 70 |
Latvija CentralPharma Communications SIA Tel: +371 6 7450497 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals and patients suitable for home treatment/self-administration
General Instructions
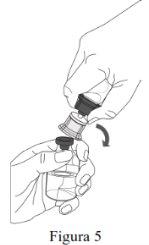
- Reconstitution must be performed according to the instructions provided below.
- The product must be reconstituted, administered, and handled with caution using aseptic techniques to maintain the sterility of the product.
- Do not use the provided sterile auxiliary devices for reconstitution and administration if their packaging is open or damaged.
- The powder must be reconstituted with the solvent (water for injectable preparations).
- Complete reconstitution of the powder must be performed within 5 minutes (1,000 mg presentation) or within 10 minutes (4,000 mg and 5,000 mg presentations).
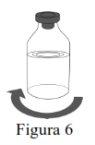
- Inspect the reconstituted solution for particles and discoloration before administration.
- The reconstituted solution must be clear, colorless, or slightly yellowish and free from visible particles.
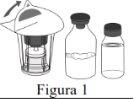
Follow the steps below for the preparation and reconstitution of Respreeza:
| |
| |
| |
Do not remove the Mix2Vial from the blister package. |
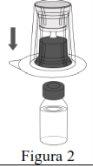
|
|
|
|
|
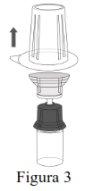
| |
| |
NOTE: Ensure that all the water has been transferred to the Respreeza vial. |
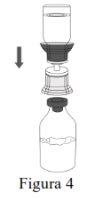
|
Discard the water for injectable preparations vial with the entire Mix2Vial. |
|
|
|
| |
Use a separate, unused Mix2Vial and water for injectable preparations vial for each Respreeza vial. | |
Use aseptic technique to transfer the reconstituted solution to the infusion container. |
Administration
The reconstituted solution should be administered using an IV infusion set (supplied with the 4,000 mg and 5,000 mg presentations).
|
|
|
|
|
|
|
|
|
Each vial of Respreeza is for single use.
Any unused product or residual material should be discarded according to the instructions of your doctor or healthcare professional.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RESPREEZA 5,000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 1000 mgActive substance: alfa1 antitrypsinManufacturer: Instituto Grifols S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 1000 mg alpha 1 antitrypsinActive substance: alfa1 antitrypsinManufacturer: Grifols Deutschland GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 4,000 mgActive substance: alfa1 antitrypsinManufacturer: Grifols Deutschland GmbhPrescription required
Online doctors for RESPREEZA 5,000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
Discuss questions about RESPREEZA 5,000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions