
PROLASTINA 1000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION


How to use PROLASTINA 1000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Prolastina 1,000 mg powder and solvent for solution for infusion
Active substance: human alpha1-antitrypsin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.,as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Prolastina and what is it used for
- What you need to know before you start using Prolastina
- How to use Prolastina
- Possible side effects
- Storage of Prolastina
- Contents of the pack and other information
1. What is Prolastina and what is it used for
Prolastina belongs to a group of medicines called "Protease Inhibitors".
Alpha1-antitrypsin is a normal component of human blood, whose function is to inhibit the activity of certain enzymes such as elastases that can damage the lungs. When there is a hereditary deficiency of alpha1-antitrypsin, there is an imbalance between alpha1-antitrypsin and elastases. This can lead to progressive destruction of lung tissue and the development of pulmonary emphysema. Pulmonary emphysema is an abnormal enlargement of the lung, accompanied by destruction of lung tissue. Prolastina is indicated to restore the balance between alpha1-antitrypsin and elastases in the lung, and consequently, to prevent further deterioration of pulmonary emphysema.
Prolastina is used as chronic treatment in certain patients with alpha1-antitrypsin deficiency as determined by their doctor.
2. What you need to know before you start using Prolastina
Do not use Prolastina
- If you are allergic (hypersensitive) to alpha1-antitrypsin or any of the other components of Prolastina (listed in section 6).
- If you have a deficiency of certain immunoglobulins, known as IgA, as severe allergic reactions may develop that can lead to anaphylactic shock.
Warnings and precautions
- Talk to your doctor, pharmacist, or nurse before starting to use Prolastina.
- Tell your doctor if you have severe heart failure (heart failure). Caution is required, as Prolastina may cause a transient increase in blood volume.
Allergic reactions (hypersensitivity)
Rarely, allergic reactions to Prolastina may occur, even if you have previously been administered human alpha1-antitrypsin inhibitors and have tolerated them well.
Your doctor will inform you about the symptoms of allergic reactions and what to do if you experience them (see also section 4).
If you experience any symptoms of an allergic reaction during the infusion of the medicine, inform your doctor or nurse immediately.
Safety information regarding the risk of infections
To prevent the transmission of infectious diseases due to the use of medicines derived from human blood or plasma, standard measures are taken, including:
- careful selection of blood and plasma donors to ensure the exclusion of donors at risk of having infections,
- testing of each donation and plasma pool to detect possible viruses or infections
- inclusion of stages in the manufacturing process to inactivate/eliminate viruses.
Despite this, when administering medicines derived from human blood or plasma, the possibility of transmitting infectious agents cannot be completely excluded. This also applies to emerging or unknown viruses and other pathogens.
These procedures are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus. The inactivation/elimination procedures may have limited value for non-enveloped viruses, such as hepatitis A virus or parvovirus B19. Infection with parvovirus B19 can have serious consequences in pregnant women (fetal infection) and in patients with immunodeficiency or increased erythropoiesis (e.g., in case of hemolytic anemia).
If you receive chronic treatment with medicines derived from human plasma (such as protease inhibitors), it is recommended that you be vaccinated against hepatitis A and B.
It is strongly recommended that each time you are administered Prolastina, the name and batch number of the medicine be recorded; this allows for the monitoring of patients who have received each batch of product.
Smoking
Since the efficacy of Prolastina is compromised by the presence of tobacco smoke in the lungs, it is recommended that patients quit smoking.
Children and adolescents
There is no experience with the use of Prolastina in children or adolescents under 18 years of age.
Other medicines and Prolastina
No interactions between Prolastina and other medicines are known.
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using a medicine.
There are no controlled clinical trials on the use of this medicine during pregnancy. Inform your doctor if you are pregnant or think you may be pregnant.
It is unknown whether Prolastina passes into breast milk, so it should not be administered to women during breastfeeding.
Consult your doctor if you are breastfeeding.
Driving and using machines
No effects on the ability to drive or use machines have been observed.
Prolastina contains sodium
Prolastina 1000 mg contains approximately 110.4 mg of sodium (main component of table/cooking salt). In the case of a 75 kg patient, the recommended dose is equivalent to 24.84% of the maximum daily sodium intake recommended for an adult. Consult your doctor or pharmacist if you have been advised to follow a low-salt diet.
3. How to use Prolastina
After reconstituting the solution with the solvent included in the package, Prolastina should be administered by intravenous infusion. A specialist doctor in chronic obstructive pulmonary disease will supervise the first infusions with Prolastina.
Home treatment
After the first infusions, a healthcare professional may also administer Prolastina, but only after receiving adequate training. If your doctor decides that you are suitable for such home treatment, they will ensure that the healthcare professional receives adequate training regarding:
- how to prepare and administer the reconstituted solution for infusion (see the illustrated instructions at the end of this leaflet,
- how to maintain the product's sterility (aseptic infusion techniques),
- how to record the treatment,
- how to identify side effects, including symptoms of allergic reactions and the measures to be taken in case they occur (see also section 4).
Dose
The amount of Prolastina administered is based on your body weight. A weekly dose of 60 mg of the active substance per kg of body weight is recommended (in the case of a 75 kg patient, this dose is equivalent to 180 ml of the reconstituted solution for infusion and contains 25 mg of alpha1-antitrypsin inhibitor (human) per ml), which is normally sufficient to maintain the protective levels of the alpha1-antitrypsin inhibitor to prevent the worsening of pulmonary emphysema.
Your doctor will indicate the duration of your treatment, as the need to limit the duration of treatment has not been established to date.
If you feel that the effect of Prolastina is too strong or too weak, talk to your doctor or pharmacist.
If you use more Prolastina than you should
To date, the effects of an overdose are unknown.
- Tell your doctor or healthcare professional if you think you have used more Prolastina than you should, so they can take the necessary measures.
- In case of overdose or accidental administration, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount administered.
If you forget to use Prolastina
- Consult your doctor if the forgotten dose should be administered.
- Do not administer a double dose to make up for the missed infusion.
If you stop using Prolastina
If you stop using Prolastina, your disease may worsen. Consult your doctor immediately if you want to stop using Prolastina.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If side effects occur during the infusion of Prolastina, the infusion should be stopped or interrupted, depending on the nature and severity of the side effect.
Serious side effects
Rarely, allergic reactions (hypersensitivity) may occur (may affect up to 1 in 1,000 people), and in some very rare cases, these reactions may occur as anaphylaxis of any type (may affect up to 1 in 10,000 people), even if you have not experienced allergy symptoms in previous infusions.
Tell your doctor or nurse immediatelyif you notice any of the following symptoms:
- rash, hives, itching,
- difficulty swallowing,
- swelling of the face or mouth,
- redness,
- difficulty breathing (dyspnea),
- low blood pressure,
- abnormal heartbeat,
- chills
Your doctor or healthcare professional will decide whether to reduce or interrupt the infusion and initiate the necessary treatment as needed.
In case of home treatment, interrupt the infusion immediatelyand contact your doctor or healthcare professional.
During treatment with Prolastina, the following side effects have been observed:
Uncommon (may affect up to 1 in 100 people):
- Chills, fever, flu-like symptoms, chest pain.
- Hives (urticaria).
- Dizziness, drowsiness, headache.
- Difficulty breathing (dyspnea).
- Skin rashes.
- Nausea.
- Joint pain (arthralgia).
Rare (may affect up to 1 in 1,000 people):
- Allergic reactions.
- Fast heart rate (tachycardia).
- Low blood pressure (hypotension).
- High blood pressure (hypertension).
- Back pain.
Very rare (may affect up to 1 in 10,000 people):
- Anaphylactic shock.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Prolastina
Do not store above 25°C.
Do not freeze.
Do not refrigerate the solution after reconstitution. The reconstituted solution must be used within 3 hours of preparation. The unused product must be discarded. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton.
6. Container Contents and Additional Information
Prolastina Composition
- The active ingredient is human alpha1-antitrypsin (derived from human blood or plasma).
- The other components are sodium chloride, sodium dihydrogen phosphate, and water for injectable preparations (solvent/diluent).
Appearance of the Product and Container Contents
Human alpha1-antitrypsin is a white or pale yellow or pale brown powder or a friable mass.
Once reconstituted with water for injectable preparations, the solution should be between transparent and slightly opalescent, colorless, pale green, pale yellow, or pale brown and free of visible particles.
1 ml of the reconstituted solution contains 25 mg of human alpha1-antitrypsin.
An individual container
Prolastina 1,000 mg powder and solvent for solution for infusion contains:
- 1 vial with powder containing 1,000 mg of human alpha1-antitrypsin.
- 1 vial with 40 ml of solvent (water for injectable preparations).
- 1 transfer device for reconstitution.
The Prolastina 1,000 mg multipack container contains:
- 4 individual boxes of Prolastina 1,000 mg.
Marketing Authorization Holder and Manufacturer
Holder:
Grifols Deutschland GmbH
Colmarer Straße 22
60528 Frankfurt
Germany
Phone: +49 69/660 593 100
Email: [email protected]
Manufacturer:
Instituto Grifols, S.A.
Can Guasch, 2 – Parets del Vallès
08150 Barcelona
Spain
Local Representative:
Instituto Grifols, S.A.
Can Guasch, 2 - Parets del Vallès
08150 Barcelona
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Prolastin 1000 mg Germany, France, Ireland
Prolastin Austria, Greece, Italy, Netherlands, Poland, Portugal
Prolastina Denmark, Finland, Norway, Spain, Sweden
Pulmolast 1000 mg Belgium
Date of the last revision of this leaflet: May 2024
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
This information is intended for healthcare professionals and patients suitable for home treatment.
Preparation of the Reconstituted Solution for Infusion:
- Use aseptic conditions (cleaning and disinfection) to maintain sterility. Reconstitute the medication on a flat work surface during the preparation of the solution.
- Ensure that the Prolastina powder vials and solvent vials (sterile water for injectable preparations) are at room temperature (20-25°C).
- Remove the protective cap from both the Prolastina vial and the solvent vial and clean the edges of the neck and the stoppers with an alcohol swab. Let the rubber stoppers dry.
|
|
|
|
|
|
- If more than one vial of product is needed to achieve the required dose, repeat the above instructions using the additional container that contains a new transfer adapter. Do not reuse the adapter.
The total dissolution should be obtained in approximately 5 minutes for the 1000 mg Prolastina presentation.
Only solutions that are between transparent and slightly opalescent, colorless, pale green, pale yellow, or pale brown and free of visible particles should be used. Prolastina should not be mixed with other infusion solutions. The reconstituted solution should always be used within 3 hours after preparation.
The reconstituted solution should be administered by slow intravenous infusion, using an infusion set (not included) suitable for this purpose. The infusion rate should be less than 0.08 ml per kg of body weight per minute (equivalent to 6 ml per minute for a 75 kg patient).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROLASTINA 1000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 1000 mgActive substance: alfa1 antitrypsinManufacturer: Instituto Grifols S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 4,000 mgActive substance: alfa1 antitrypsinManufacturer: Grifols Deutschland GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 5,000 mgActive substance: alfa1 antitrypsinManufacturer: Grifols Deutschland GmbhPrescription required
Online doctors for PROLASTINA 1000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION
Discuss questions about PROLASTINA 1000 mg POWDER AND SOLVENT FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





 Open the sterile package of the transfer device by completely removing the cap. Do not remove the device from its wrapper.
Open the sterile package of the transfer device by completely removing the cap. Do not remove the device from its wrapper.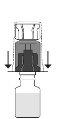 Place the solvent vial in a vertical position on the flat surface and hold it firmly. Without removing the outer wrapper, press the blue terminal of the transfer adapter downwards until the tip pierces the stopper and fits. Avoid twisting it.
Place the solvent vial in a vertical position on the flat surface and hold it firmly. Without removing the outer wrapper, press the blue terminal of the transfer adapter downwards until the tip pierces the stopper and fits. Avoid twisting it.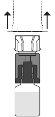
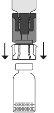 Place the Prolastina powder vial in a vertical position on the surface. Turn the unit formed by the adapter and the solvent vial upside down 180°. Push it with the transparent/white terminal of the adapter straight downwards, without twisting, until the tip pierces the stopper and fits.
Place the Prolastina powder vial in a vertical position on the surface. Turn the unit formed by the adapter and the solvent vial upside down 180°. Push it with the transparent/white terminal of the adapter straight downwards, without twisting, until the tip pierces the stopper and fits.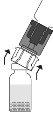 Due to the vacuum in the powder vial, the transfer of solvent will start automatically. Wait for the transfer of solvent to be completed. Remove the adapter with the solvent vial connected at an approximate angle of 45°.
Due to the vacuum in the powder vial, the transfer of solvent will start automatically. Wait for the transfer of solvent to be completed. Remove the adapter with the solvent vial connected at an approximate angle of 45°. Gently turn the Prolastina vial until the powder is completely dissolved. Do not shake to avoid foam formation. Do not touch the stopper. Administer the product using an aseptic technique.
Gently turn the Prolastina vial until the powder is completely dissolved. Do not shake to avoid foam formation. Do not touch the stopper. Administer the product using an aseptic technique.









