

Rimphisia

Ask a doctor about a prescription for Rimphisia

How to use Rimphisia
Leaflet accompanying the packaging: information for the user
Rymphysia, 500 mg, powder and solvent for solution for infusion
Rymphysia, 1000 mg, powder and solvent for solution for infusion
Human alpha1-proteinase inhibitor
This medicinal product is subject to additional monitoring. This will allow for quick identification of new safety information. The user of the medicinal product can also help by reporting any adverse reactions that occur after taking the medicinal product. To find out how to report adverse reactions, see section 4.
Read the leaflet carefully before taking the medicinal product, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor or a medical professional.
- This medicinal product has been prescribed specifically for you. Do not pass it on to others. The medicinal product may harm another person, even if their symptoms are the same.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform their doctor or another medical professional. See section 4.
Table of contents of the leaflet
- 1. What is Rymphysia and what is it used for
- 2. Important information before taking Rymphysia
- 3. How to take Rymphysia
- 4. Possible adverse reactions
- 5. How to store Rymphysia
- 6. Contents of the packaging and other information
1. What is Rymphysia and what is it used for
What is Rymphysia
This medicinal product contains the active substance human alpha1-proteinase inhibitor, which is a normal component of human blood and is found in the lungs. Its main function in the lungs is to protect lung tissue by inhibiting the action of a certain enzyme called neutrophil elastase. Neutrophil elastase can cause damage if its action is not controlled (for example, if the patient has a deficiency of alpha1-proteinase inhibitor).
What is Rymphysia used for
This medicinal product is used in adults with a diagnosed severe deficiency of alpha1-proteinase inhibitor (also known as alpha1-antitrypsin deficiency), who have developed a lung disease called pulmonary emphysema. Pulmonary emphysema occurs when the lack of alpha1-proteinase inhibitor leads to a state in which neutrophil elastase is not properly controlled, causing damage to the small air sacs in the lungs through which oxygen enters the body. Due to this damage, the lungs do not function properly. Regular use of this medicinal product increases the concentration of alpha1-proteinase inhibitor in the blood and lungs, thereby protecting the lungs, limiting the action of neutrophil elastase, and thus slowing the progression of pulmonary emphysema. It should not be expected that the medicinal product will reverse the damage that has already occurred.
2. Important information before taking Rymphysia
When not to take Rymphysia
If any of the above applies to the patient, they should not take Rymphysia.
Warnings and precautions
Before starting treatment with Rymphysia, the patient should discuss it with their doctor or another medical professional.
Information on allergic reactions
The patient may be allergic to Rymphysia, even if they have previously taken similar medicinal products and tolerated them well.
- In some cases, a severe allergic reaction may occur.
- The patient will be informed by their doctor about the symptoms of allergic reactions (such as chills, flushing, rapid heartbeat, low blood pressure, feeling dizzy, rash, hives, itching, difficulty breathing or swallowing, as well as swelling of the hands, face, or mouth) - see also "Allergic reactions" in section 4.
- If the reaction is severe, the doctor may decide to reduce the infusion rate or discontinue the infusion altogether. Then, they may administer appropriate treatment for the symptoms of the allergic reaction.
The patient should immediately inform their doctor or another medical professional if they observe any of the above reactions during the infusion. If the patient is being treated at home and/or is self-administering, they should immediately discontinue the infusion and contact their doctor or another medical professional.
Risk of infection
Rymphysia is produced from human blood plasma (the liquid component of blood, from which blood cells have been removed). When medicinal products are produced from human blood or plasma, certain precautions are taken to prevent the transmission of infections to patients. These include:
- careful selection of blood and plasma donors to ensure the exclusion of donors who may be carriers of infections,
- testing of each donated sample and plasma pool for signs of viruses/infections,
- inclusion in the processing of blood or plasma of steps aimed at inactivating or removing viruses.
However, despite these precautions, it cannot be completely ruled out that the transmission of an infection may occur when administering medicinal products prepared from human blood or plasma. This also applies to any unknown or new viruses or other types of infections. The measures taken are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, as well as non-enveloped viruses such as hepatitis A virus and parvovirus B19. To improve the identification of biological medicinal products, the name and batch number of the administered product should be clearly recorded. If the patient is regularly/repeatedly administered proteinase inhibitors from human plasma, their doctor may recommend that they consider vaccination against viral hepatitis A and B. Smoking tobacco is a significant risk factor for the development and progression of pulmonary emphysema, so it is strongly recommended to quit smoking and avoid passive smoking.
Children and adolescents
This medicinal product is not intended for use in children and adolescents under the age of 18.
Rymphysia and other medicinal products
The patient should inform their doctor or another medical professional about all medicinal products they are currently taking or have recently taken, as well as any medicinal products they plan to take. This includes medicinal products that are available without a prescription and herbal medicinal products. This is necessary because Rymphysia may affect the action of certain other medicinal products. Additionally, some medicinal products may affect the action of Rymphysia.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or another medical professional before taking this medicinal product. Since alpha1-proteinase inhibitor is a normal component of human blood, it is not expected that the recommended dose of this medicinal product will have a harmful effect on the developing fetus. However, due to the lack of available data on the safety of Rymphysia during pregnancy, if the patient is pregnant, this medicinal product should only be used with caution. It is not known whether Rymphysia passes into human milk. If the patient is breastfeeding, their doctor will discuss the risks and benefits of taking this medicinal product. There are no data on the effects on fertility, but since alpha1-proteinase inhibitor is a normal component of human blood, it is not expected to have any undesirable effects on fertility when Rymphysia is used at the recommended dose.
Driving and using machines
Rymphysia may have a minor effect on the ability to drive and use tools or machines. If the patient experiences dizziness or symptoms of fatigue, they should not drive or use tools or machines.
Rymphysia contains sodium
Rymphysia, 500 mg, powder and solvent for solution for infusion:
- The medicinal product contains 108 mg of sodium (the main component of common salt) in each vial of 25 ml solution. This corresponds to 5.4% of the maximum recommended daily intake of sodium in the diet for adults.
Rymphysia, 1000 mg, powder and solvent for solution for infusion:
- The medicinal product contains 216 mg of sodium (the main component of common salt) in each vial of 50 ml solution. This corresponds to 10.8% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to take Rymphysia
Rymphysia is administered by intravenous infusion. The first infusions of the medicinal product will be supervised by a medical professional with experience in the treatment of alpha1-proteinase inhibitor deficiency.
Treatment at home and/or self-administration by the patient
After the first infusions, Rymphysia may be administered by the patient themselves or their caregiver, but only after receiving appropriate training. If the doctor decides that the patient can receive treatment at home and/or self-administer, they will train the patient or caregiver in the following:
- how to prepare and administer the medicinal product (see the instructions at the end of this leaflet),
- how to maintain the medicinal product in a sterile state (principles of aseptic infusion),
- how to keep a treatment diary,
- how to recognize adverse reactions, including symptoms of allergic reactions and measures to be taken in case of such reactions (see also section 2 and section 4).
The doctor or another medical professional will regularly check the patient's and/or caregiver's infusion technique to ensure that the medicinal product is always properly prepared and administered.
Dose
The dose of Rymphysia administered to the patient depends on their body weight. The recommended dose is 60 mg per kilogram of body weight, which should be administered once a week. The infusion will last approximately 15-30 minutes. The doctor will determine the appropriate dose and infusion rate based on the patient's body weight and tolerance of the infusion.
Overdose of Rymphysia
If the patient thinks they have taken too much Rymphysia, they should contact their doctor or another medical professional, who will decide on the appropriate course of action. The consequences of overdose are unknown.
Missed dose of Rymphysia
If an infusion is missed, the patient should take the next dose immediately and continue taking the medicinal product at the regular intervals prescribed by their doctor or another medical professional. The patient should not take a double dose to make up for the missed dose.
Discontinuation of Rymphysia
The patient should not stop taking this medicinal product without consulting their doctor or another medical professional. If treatment with Rymphysia is discontinued, the patient's condition may worsen.
4. Possible adverse reactions
Like all medicinal products, Rymphysia can cause adverse reactions, although not everybody gets them. Such adverse reactions may occur even if the patient has previously taken similar medicinal products and tolerated them well. The following adverse reactions may occur with Rymphysia:
Allergic reactions
The patient should immediately inform their doctor or another medical professional if they observe any of the following severe adverse reactions during the infusion, as they may require immediate medical attention:
- chills or flushing,
- rapid heartbeat, low blood pressure, or feeling dizzy,
- rash, hives, or itching,
- difficulty breathing or swallowing, as well as swelling of the hands, face, or mouth.
The patient should immediately inform their doctor or another medical professional if they observe any of the above severe adverse reactions. If the reaction is severe, the doctor or another medical professional may decide to reduce the infusion rate or discontinue the infusion altogether. Then, they may administer appropriate treatment for the symptoms of the allergic reaction. If the patient is being treated at home and/or is self-administering, they should immediately discontinue the infusion and contact their doctor or another medical professional.
- Allergic reactions are uncommonand may occur in up to 1 in 100 patients.
- Severe allergic reactions are rareand may occur in up to 1 in 10,000 patients.
- Allergic reactions may occur even if no symptoms of allergy have appeared in previous infusions.
Other adverse reactions
The patient should immediately inform their doctor or another medical professional if they observe any of the following adverse reactions:
Very common:may occur in more than 1 in 10 patients
- bronchitis (persistent cough that may produce mucus, wheezing, difficulty breathing);
- upper respiratory tract infection (common cold, flu-like illness);
- headache;
- cough;
- sore throat (throat and larynx pain);
Common:may occur in up to 1 in 10 patients
- altered sense of taste (taste disturbance);
- discomfort in the head, migraine;
- drowsiness;
- eye disorders (conjunctivitis, eye irritation, and vision disturbances);
- runny nose (rhinitis);
- throat irritation;
- bloating, diarrhea, mouth ulcers, vomiting;
- chest pain or discomfort;
- fever and malaise (flu-like illness);
- pain or discomfort at the infusion site;
- feeling very tired and sleepy;
- discomfort, pain, or tenderness of muscles (muscle pain);
- general feeling of discomfort, illness, or unease - without a clear reason (malaise);
Frequency not known:frequency cannot be estimated from the available data
- feeling weak;
- feeling unwell;
- reaction at the infusion site, such as bruising, pain, and rash;
Reporting adverse reactions
If adverse reactions occur, including any adverse reactions not listed in this leaflet, the patient should inform their doctor or another medical professional. Adverse reactions can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Adverse reactions can also be reported to the marketing authorization holder. By reporting adverse reactions, more information can be collected on the safety of the medicinal product.
5. How to store Rymphysia
The medicinal product should be stored out of sight and reach of children. Do not use this medicinal product after the expiry date stated on the outer packaging and on the vial labels after "EXP". The expiry date refers to the last day of the month stated. Do not store above 25°C. Do not freeze. Store in the original packaging to protect from light. After reconstitution, the resulting solution should be used immediately. If this is not possible, the solution can be stored at room temperature (up to 25°C) for up to 3 hours. The prepared solution should not be frozen.
6. Contents of the packaging and other information
What Rymphysia contains
- Active substanceis human alpha1-proteinase inhibitor. One vial contains approximately 500 mg or 1000 mg of human alpha1-proteinase inhibitor.
- Other ingredientsare: sodium chloride, sodium dihydrogen phosphate monohydrate, and sodium hydroxide.
- Solvent: sterile water for injections.
What Rymphysia looks like and what the packaging contains
Powder and solvent for solution for infusion. The medicinal product is a white or off-white to light yellow-green powder. After reconstitution with water for injections, the solution should be clear, colorless, or slightly yellow to yellow-green and should not contain any visible particles. One packaging contains:
Rymphysia, 500 mg, powder and solvent for solution for infusion:
- One vial of Rymphysia 500 mg powder
- One vial of solvent with 25 ml of sterile water for injections
- One sterile double-ended transfer needle
- One sterile filter with a pore size of 20 microns
Rymphysia, 1000 mg, powder and solvent for solution for infusion:
- One vial of Rymphysia 1000 mg powder
- One vial of solvent with 50 ml of sterile water for injections
- One sterile double-ended transfer needle
- One sterile filter with a pore size of 20 microns
Marketing authorization holder
Takeda Pharma Sp. z o.o., ul. Prosta 68, 00-838 Warsaw, Tel.: +48 22 306 24 47, [email protected]
Manufacturer
Takeda Manufacturing Austria AG, Industriestrasse 67, 1221 Vienna, Austria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Italy, Latvia, Luxembourg, Norway, Poland, Portugal, Romania, Spain, Sweden: Rymphysia
Date of last revision of the leaflet: 02/2023.
Information intended for healthcare professionals and trained caregivers and/or patients:
General instructions
- Rymphysia is intended for intravenous use only.
- Reconstitution, administration, and any handling of the product should be carried out with caution and asepsis to maintain the sterility of the product.
- Before reconstitution, allow Rymphysia and the solvent to reach room temperature.
- After reconstitution, Rymphysia should be stored at room temperature and administered within three (3) hours of reconstitution. Partially used vials should be discarded and not kept for future use. The solution does not contain a preservative.
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Reconstitution
- 1. Remove the caps from the solvent and product vials.
- 2. Wipe the exposed surfaces of the stoppers with alcohol.
- 3. Remove the protector from one end of the double-ended transfer needle. Insert the exposed end of the needle through the center of the solvent vial stopper.
- 4. Remove the plastic protector from the other end of the double-ended transfer needle, which is embedded in the solvent vial stopper. To minimize foaming, invert the solvent vial and insert the exposed end of the needle through the center of the product vial stopper at an angle, ensuring that the solvent vial is always above the product vial. Insertion at an angle directs the solvent stream to the vial wall. See the diagram below. The vacuum in the vial is sufficient to transfer all of the solvent.
- 5. Separate the two vials by removing the solvent vial from the transfer needle. This equalizes any negative pressure that may have developed in the product vial. Then, remove the double-ended transfer needle from the product vial and discard the needle into an appropriate sharps container.
- 6. Stand the vial upright and allow the solution to dissolve, then GENTLY rotate the vial to ensure complete dissolution of the powder. Reconstitution takes no more than five (5) minutes for the 500 mg vial and no more than ten (10) minutes for the 1000 mg vial. Note: Do not shake the vial contents. The vials should not be inverted until ready for withdrawal.
- 7. The reconstituted product is colorless or slightly yellow to yellow-green.
- 8. Occasionally, a few small visible particles may remain in the reconstituted product. These will be removed by the sterile filter with a pore size of 20 microns provided with the product.
Administration
- 1. Before administration, inspect the reconstituted product for particulate matter and discoloration.
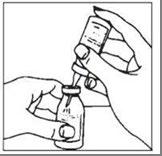
- 2. The contents of several vials can be transferred to an empty, sterile container for intravenous solutions. This should be done using aseptic technique and a sterile needle that is not provided with the product.
- 3. To minimize the risk of developing harmful microorganisms, Rymphysia should be administered within three (3) hours of reconstitution. Any unused solution should be discarded.
- 4. Rymphysia should be administered separately, without mixing with any other agents or diluents.
Infusion rate
- The recommended infusion rate is 0.08 ml/kg body weight per minute. The infusion rate can be adjusted based on the patient's clinical response and tolerance, but should not exceed 0.2 ml/kg body weight per minute.
- If adverse reactions occur, the infusion rate should be reduced or the infusion discontinued. After the symptoms have resolved, the infusion can be resumed at a rate tolerated by the patient.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterTakeda Manufacturing Austria AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RimphisiaDosage form: Powder, 1000 mgActive substance: alfa1 antitrypsinManufacturer: Takeda Manufacturing Austria AGPrescription requiredDosage form: Solution, 277.8 Ph. Eur. units (500,000 KIU)Active substance: aprotininPrescription not requiredDosage form: Tablets, 500 mgActive substance: tranexamic acidPrescription required
Alternatives to Rimphisia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Rimphisia in Spain
Alternative to Rimphisia in Ukraine
Online doctors for Rimphisia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Rimphisia – subject to medical assessment and local rules.














