
REQUIP-PROLIB 4 mg PROLONGED-RELEASE TABLETS

How to use REQUIP-PROLIB 4 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
REQUIP-PROLIB 4 mg prolonged-release tablets
ropinirole
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet.See section 4.
Contents of the pack
- What REQUIP-PROLIB is and what it is used for
- What you need to know before you take REQUIP-PROLIB
- How to take REQUIP-PROLIB
- Possible side effects
- Storage of REQUIP-PROLIB
- Contents of the pack and other information
1. What REQUIP-PROLIB is and what it is used for
The active substance of REQUIP-PROLIB is ropinirole, which belongs to a group of medicines called dopamine agonists. Dopamine agonists work in a similar way to a natural substance called dopamine in the brain.
REQUIP-PROLIB prolonged-release tablets are used to treat Parkinson's disease.
People with Parkinson's disease have low levels of dopamine in some parts of their brain. Ropinirole has a similar effect to natural dopamine and reduces the symptoms of Parkinson's disease.
2. What you need to know before you take REQUIP-PROLIB
Do not take REQUIP-PROLIB
- if you are allergicto ropinirole or any of the other ingredients of this medicine (listed in section 6)
- if you have severe kidney disease
- if you have severe liver disease
- Tell your doctor if you think you have any of these conditions.
Warnings and precautions
Talk to your doctor or pharmacist before taking REQUIP-PROLIB:
- if you are pregnantor think you may be pregnant
- if you are breast-feeding
- if you are under 18 years old
- if you have severe heart disease
- if you have severe mental health problems
- if you have impulsive or abnormal behaviour(see section 4)
- if you have intolerance to some sugars(e.g. lactose).
Tell your doctor if you notice symptoms such as depression, apathy, anxiety, fatigue, sweating or painwhen stopping or reducing treatment with REQUIP-PROLIB (called dopamine agonist withdrawal syndrome or DAWS). If problems persist after a few weeks, your doctor may need to adjust your treatment.
Tell your doctor if you or your family/carer notice that you are developing impulses or desires to behave in ways that are unusual for you and that you cannot resist the impulse, desire or temptation to perform certain activities that could harm yourself or others. This is called impulse control disorders and can include behaviours such as pathological gambling, excessive eating or spending, abnormally high sexual desire or an increase in sexual thoughts or feelings. Your doctor may need to adjust or stop your treatment.
Tell your doctor if you or your family/carer notice that you are developing episodes of overactivity, euphoria or irritability (symptoms of mania). These can occur with or without symptoms of impulse control disorders (see above). Your doctor may need to adjust or stop your treatment.
Tell your doctorif you think you are in any of these situations. Your doctor will decide if your treatment with REQUIP-PROLIB is suitable for you, or if you need any additional monitoring while taking it.
While taking REQUIP-PROLIB
Tell your doctor if you or your family notice that you are developing any abnormal behaviour(such as an abnormal need to gambleor an increase in sexual desires and/or behaviour) while taking REQUIP-PROLIB. Your doctor may need to adjust or stop your treatment.
Smoking and REQUIP-PROLIB
Tell your doctorif you start or stop smoking while taking REQUIP-PROLIB. Your doctor may need to adjust your dose.
Other medicines and REQUIP-PROLIB
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
Remember to tell your doctoror pharmacist if you start taking a new medicine while taking REQUIP-PROLIB.
Some medicines may affect the way REQUIP-PROLIB works, or make it more likely that you will have side effects. REQUIP-PROLIB may also affect the way other medicines work.
These medicines include:
- the antidepressantfluvoxamine
- medicines for mental health problems, such as sulpiride
- hormone replacement therapy (also called HRT)
- metoclopramide, used to treat nausea and stomach acid
- the antibioticsciprofloxacinor enoxacin
- any other medicine for Parkinson's disease
Tell your doctorif you are taking or have taken any of these medicines.
If you are taking the following medicines with REQUIP-PROLIB you may need to have additional blood tests:
- vitamin K antagonists (used to prevent blood clotting) such as warfarin.
Taking REQUIP-PROLIB with food and drink
You can take REQUIP-PROLIB with or without food, as you prefer.
Pregnancy and breast-feeding
REQUIP-PROLIB should not be used during pregnancy
Tell your doctor immediatelyif you are pregnant, think you may be pregnant, or plan to become pregnant. Your doctor will advise you whether to stop taking REQUIP-PROLIB if you are breast-feeding or plan to breast-feed.
Driving and using machines
REQUIP-PROLIB may make you feel drowsy. Some people may feel extremely drowsyor fall asleep suddenly without warning.
REQUIP-PROLIB may cause hallucinations (seeing, hearing or feeling things that are not there). If this happens, do not drive or use machines.
If you experience this: do not drive, do not use machines, and do notput yourself in situations where feeling drowsy or falling asleep could put you or others at risk of serious injury or death. Do not do these activities until you are no longer affected.
Ask your doctorif this could affect you.
REQUIP-PROLIBcontains lactose, sunset yellow, sodium and hydrogenated castor oil
This medicine contains lactose. If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
This medicine may cause allergic reactions because it contains sunset yellow FCF (E110).
It may cause asthma, especially in patients allergic to acetylsalicylic acid.
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; this is, essentially, “sodium-free”.
This medicine may cause stomach upset and diarrhea because it contains hydrogenated castor oil.
3. How to take REQUIP-PROLIB
Take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist again.
Do not give REQUIP-PROLIB to children. REQUIP-PROLIB is not normally prescribed for people under 18 years old.
You may have been prescribed REQUIP-PROLIB on its own to treat the symptoms of your Parkinson's disease, or you may have been prescribed REQUIP-PROLIB as well as another medicine called L-dopa (also known as levodopa). If you are taking L-dopa, you may experience some uncontrolled movements (dyskinesias) when you first start taking REQUIP-PROLIB. Tell your doctor if this happens, as they may need to adjust the doses of the medicines you are taking.
The prolonged-release tablets of REQUIP-PROLIB are designed to release the active substance over a 24-hour period. If you are in a state where medicines pass through your body very quickly, for example with diarrhea, the tablet may not dissolve completely and may not work properly. You may see tablets in your stools. If this happens, tell your doctor as soon as possible.
What dose of REQUIP-PROLIB should you take?
It may take some time to find the best dose of REQUIP-PROLIB for you.
The recommended starting doseof REQUIP-PROLIB prolonged-release tablets is 2 mg once a day for the first week. From then on, your doctor may increase your dose to 4 mg of REQUIP-PROLIB prolonged-release tablets once a day, during the second week of treatment. In elderly people, the doctor may increase the dose more slowly. After that, your doctor may adjust your dose until they find the best dose for you. Some people take up to 24 mg of REQUIP-PROLIB prolonged-release tablets each day.
If you experience side effects that you cannot tolerate when you first start treatment, talk to your doctor. Your doctor may advise you to switch to a lower dose of REQUIP film-coated tablets (immediate release) that you take three times a day.
Do not take more REQUIP-PROLIB tablets than your doctor has recommended.
It may take several weeks for REQUIP-PROLIB to start working.
How to take your dose of REQUIP-PROLIB
Take REQUIP-PROLIB once a day, at the same time each day.
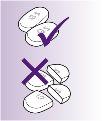
Swallow the REQUIP-PROLIB tablets whole, with a glass of water.
Do not break, chew or crush the prolonged-release tablets. If you do, you may be at risk of receiving a high dose, because the medicine will be released into your body too quickly.
If you switch from REQUIP film-coated tablets (immediate release)
Your doctor will adjust your dose of REQUIP-PROLIB prolonged-release tablets based on the dose of REQUIP film-coated tablets (immediate release) you are taking.
Take your REQUIP film-coated tablets (immediate release) as usual the day before you switch. Then, take your REQUIP-PROLIB prolonged-release tablets the next morning, and do not take any more REQUIP film-coated tablets (immediate release).
If you take more REQUIP-PROLIB than you should
Contact your doctor or pharmacist immediately. If possible, show them the REQUIP-PROLIB pack.
Someone who has taken too much REQUIP-PROLIB may experience some of the following symptoms: nausea, vomiting, dizziness, drowsiness, fatigue (mental or physical), feeling faint, hallucinations.
If you forget to take REQUIP-PROLIB
Do not take extra tablets or a double dose to make up for forgotten doses.
If you have forgotten to take REQUIP-PROLIB for one or more days, talk to your doctor to find out how to start taking it again.
If you stop taking REQUIP-PROLIB
Do not stop taking REQUIP-PROLIB without talking to your doctor first.
Take REQUIP-PROLIB for as long as your doctor recommends. Do not stop taking it unless your doctor tells you to.
If you stop taking REQUIP-PROLIB suddenly, your Parkinson's disease symptoms may quickly get worse. Stopping treatment suddenly may cause you to develop a condition called neuroleptic malignant syndrome, which can be life-threatening. The symptoms include: akinesia (loss of muscle movement), muscle stiffness, fever, unstable blood pressure, rapid heart rate, confusion, decreased level of consciousness (e.g. coma).
If you need to stop taking REQUIP-PROLIB, your doctor will reduce your dose gradually.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medications, this medication can produce adverse effects, although not all people suffer from them.
Adverse reactions to REQUIP-PROLIB usually appear with greater probability at the start of treatment or when increasing the dose. Adverse reactions are generally mild and may be less bothersome after a while. Inform your doctor if you are concerned about the adverse effects.
Very Common Adverse Effects
These may affect more than 1 in 10 peoplewho take REQUIP-PROLIB:
- fainting
- drowsiness
- discomfort (nausea)
Common Adverse Effects
These may affect up to 1 in 10 peoplewho take REQUIP-PROLIB:
- sudden onset of sleep without prior feeling of sleepiness (sudden sleep episodes)
- hallucinations (seeing things that are not really there)
- vomiting
- dizziness (feeling of spinning)
- heartburn
- stomach pain
- constipation
- swelling of the legs, feet, or hands
Uncommon Adverse Effects
These may affect up to 1 in 100 peoplewho take REQUIP-PROLIB:
- dizziness or fainting, especially when standing up suddenly (due to a drop in blood pressure)
- low blood pressure (hypotension)
- feeling very drowsy during the day (excessive somnolence)
- mental problems such as delirium (severe confusion), delusional ideas (irrational ideas), or paranoia (irrational suspicions)
- hypo
Some patients may experience the following adverse effects (frequency not known: cannot be estimated from the available data)
- allergic reactions such as redness, skin inflammation with itching (hives), swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing, rash, or intense itching (see section 2)
- changes in liver function, which can be detected with blood tests
- acting aggressively
- overuse of REQUIP-PROLIB (anxiety about taking an excessive dose of dopaminergic medications regarding the necessary dose to control motor symptoms, known as dopamine dysregulation syndrome)
- inability to resist the impulse, desire, or temptation to perform certain actions that may be harmful to you or others, which may include:
- strong impulse to gamble excessively, despite significant personal or family consequences
- altered or increased sexual interest and behavior, and very worrying behavior for you or others, such as exaggerated sexual behavior
- uncontrollable shopping or spending
- binge eating (eating large amounts of food in a short period) or compulsive eating (eating more food than necessary to satisfy hunger)
- episodes of overactivity, euphoria, or irritability
- after stopping or reducing treatment with REQUIP-PROLIB, depression, apathy, anxiety, fatigue, sweating, or pain may occur (known as dopamine agonist withdrawal syndrome or DAWS)
- spontaneous penile erection
Tell your doctor if you experience any of these behaviors; they will indicate ways to manage or reduce the symptoms.
If you are taking REQUIP-PROLIB with L-dopa
People who take REQUIP-PROLIB with L-dopa may develop other adverse effects after a while:
- very common adverse effects are uncontrolled movements (dyskinesias). If you are taking L-dopa, you may experience some uncontrolled movements (dyskinesias) when you start taking REQUIP-PROLIB. Inform your doctor if this occurs, as you may need to adjust the doses of the medications you are taking
- a common adverse effect is confusion
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of REQUIP-PROLIB
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the blister pack and packaging. The expiration date is the last day of the month indicated.
Do not store above 25°C. Store in the original packaging to protect it from light.
Medications should not be thrown away through the drains or in the trash. Deposit the packaging and medications you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of REQUIP-PROLIB
The active ingredient of REQUIP-PROLIB is ropinirole
One prolonged-release tablet contains 4 mg of ropinirole (as hydrochloride)
The other components are:
- Prolonged-release tablet core:hypromellose, hydrogenated ricin oil, sodium carmellose, povidone (K29-32), maltodextrin, magnesium stearate, lactose monohydrate, anhydrous colloidal silica, mannitol (E421), yellow iron oxide (E172), glycerol dibehenate
- Coating film: OPADRY light brown OY-27207 (hypromellose, titanium dioxide (E171), macrogol 400, yellow aluminum lake sunset FCF (E110), aluminum lake carmine indigo (E132))
Appearance of the Product and Package Contents
REQUIP-PROLIB 4 mg is presented in the form of light brown, capsule-shaped, prolonged-release tablets, engraved with "GS" on one side and "WXG" on the other
REQUIP-PROLIB 4 mg is supplied in packages with blister packs of 28 or 84 prolonged-release tablets
Only some package sizes may be marketed
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder | GlaxoSmithKline, S.A P.T.M. C/ Severo Ochoa, 2 28760 Tres Cantos (Madrid) Tel: +34 900 202 700 |
Manufacturer | Glaxo Wellcome S.A Avenida de Extremadura 3 09400 Aranda de Duero Burgos |
Procedure Number: FR/H/111/008
This medication is authorized in the member states of the European Economic Area with the following names:
Germany, Austria, Belgium, Ireland, Luxembourg, Netherlands: Requip modutab
Finland and Sweden: Requip Depot
France and Portugal: Requip LP
Greece: Requip XL
Italy: Requip
Spain: Requip Prolib
Date of the Last Revision of this Prospectus:June 2023
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price17.98 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REQUIP-PROLIB 4 mg PROLONGED-RELEASE TABLETSDosage form: TABLET, 0.25 mgActive substance: ropiniroleManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: TABLET, 0.5 mgActive substance: ropiniroleManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: TABLET, 2 mgActive substance: ropiniroleManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for REQUIP-PROLIB 4 mg PROLONGED-RELEASE TABLETS
Discuss questions about REQUIP-PROLIB 4 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








