REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSION

Ask a doctor about a prescription for REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSION

How to use REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSION
Introduction
Package Leaflet: Information for the Patient
REKAMBYS 900 mg prolonged-release injectable suspension
rilpivirine
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is REKAMBYS and what is it used for
- What you need to know before you use REKAMBYS
- How REKAMBYS is administered
- Possible side effects
- Storage of REKAMBYS
- Contents of the pack and further information
1. What is REKAMBYS and what is it used for
REKAMBYS contains the active substance rilpivirine. It belongs to a group of medicines called non-nucleoside reverse transcriptase inhibitors (NNRTIs) used to treat human immunodeficiency virus type 1 (HIV-1).
REKAMBYS works with other HIV medicines to block the ability of the virus to make more copies of itself. REKAMBYS injectable does not cure HIV infection, but it helps to reduce the amount of HIV in your body and keep it at a low level. This helps to delay damage to your immune system and the development of infections and diseases associated with AIDS.
REKAMBYS is always given with another HIV medicine called cabotegravir injectable. They are given together in adults from 18 years of age whose HIV-1 infection is already controlled.
2. What you need to know before you use REKAMBYS
Do not use REKAMBYSif you are allergic to rilpivirine or any of the other ingredients of this medicine (listed in section 6).
Do not use REKAMBYS if you are taking any of the following medicines, as they may affect how REKAMBYS or the other medicines work:
- carbamazepine, oxcarbazepine, phenobarbital, phenytoin (medicines to treat epilepsy and prevent seizures)
- rifabutin, rifampicin, rifapentine (medicines to treat bacterial infections such as tuberculosis)
- dexamethasone (a corticosteroid used to treat various conditions, such as inflammation and allergic reactions) given in a treatment cycle by mouth or by injection
- products containing St. John's Wort or Hypericum (Hypericum perforatum, a medicinal plant used for depression).
If you are taking any of these medicines, ask your doctor about alternatives.
Warnings and precautions
Talk to your doctor or pharmacist before you start using REKAMBYS.
REKAMBYS does not cure HIV infection. It is part of a treatment to reduce the amount of virus in the blood. While you are using this medicine, you can still transmit HIV to others, although the risk is reduced by effective antiviral treatment. Ask your doctor about the precautions you should take to avoid infecting others.
Tell your doctor about your situation
Check the following points and tell your doctor if you are in any of these situations.
- You must attend all scheduled visits for injections. Do not miss any visits, it is very important for the success of your treatment. If you cannot attend a scheduled visit, inform your doctor as soon as possible.
- Tell your doctor if you have or have ever had liver disease, including hepatitis B or C, or kidney disease. Your doctor will probably check how well your liver and kidneys are working to decide if you can use REKAMBYS. Check the signs of liver damage in section 4 of this leaflet “Uncommon side effects”.
- Tell your doctor immediately if you notice any symptoms of infection(e.g. fever, chills, sweats). In some patients with HIV, inflammation may occur due to previous infections shortly after starting HIV treatment. These symptoms are thought to be due to the improvement of the body's immune response, which allows it to fight infections that were previously present but did not show obvious symptoms.
- Tell your doctor immediately if you notice any symptoms such as muscle weakness, weakness starting in hands and feet and moving up to the body, palpitations, tremors, or hyperactivity. This is due to autoimmune disorders (conditions in which the immune system attacks healthy body tissue by mistake) that can also occur after you start taking medicines for the treatment of your HIV infection. Autoimmune disorders can appear many months after starting treatment.
- Tell your doctor if you are taking any medicine that you have been told may cause an irregular heartbeat that can be potentially fatal (Torsades de Pointes).
Injection site reactions
Some people have experienced symptoms of injection site reactions within a few minutes of receiving the rilpivirine injection. Most symptoms resolved a few minutes after the injection. Injection site reaction symptoms may include: difficulty breathing, stomach cramps, sweating, numbness of the mouth, feeling anxious, feeling hot, feeling dizzy or faint, and changes in blood pressure. Tell your healthcare professional if you experience these symptoms after receiving your injections.
It is important to attend scheduled visits
It is very important that you attend your scheduled visitsto receive REKAMBYS to control your HIV infection and to prevent the disease from getting worse. Do not miss any visits, it is very important for the treatment to be effective. If you cannot attend a scheduled visit, inform your doctor as soon as possible. Tell your doctor if you are thinking of stopping treatment. If REKAMBYS administration is delayed, or if you stop receiving REKAMBYS, you will need to take other medicines to treat your HIV infection and reduce the risk of the virus becoming resistant, as the levels of medicine in your body will be too low to treat the HIV infection.
Children
REKAMBYS is not for use in children or adolescents under 18 years of age, as it has not been studied in these patients.
Other medicines and REKAMBYS
Tell your healthcare professional if you are taking, have recently taken, or might take any other medicines. Some medicines may affect the levels of REKAMBYS in the blood if taken during treatment with REKAMBYS, or REKAMBYS may affect the effectiveness of other medicines.
Do not use REKAMBYS if you are taking any of the following medicines, as they may affect how REKAMBYS or the other medicines work:
- carbamazepine, oxcarbazepine, phenobarbital, phenytoin (medicines to treat epilepsy and prevent seizures)
- rifabutin, rifampicin, rifapentine (medicines to treat bacterial infections such as tuberculosis)
- dexamethasone (a corticosteroid used to treat various conditions, such as inflammation and allergic reactions) given in a treatment cycle by mouth or by injection
- products containing St. John's Wort or Hypericum (Hypericum perforatum, a medicinal plant used for depression).
If you are taking any of these medicines, ask your doctor about alternatives.
The effect of REKAMBYS or other medicines may changeif you use REKAMBYS with any of the following medicines:
- clarithromycin, erythromycin (antibiotics)
- methadone (used to treat withdrawal symptoms and dependence)
Pregnancy and breastfeeding
Tell your doctor immediately if you are pregnant or plan to become pregnant. Your doctor will assess the benefits and risks to you and your baby of using REKAMBYS during pregnancy. If you plan to become pregnant, talk to your doctor first, as rilpivirine may remain in your body for up to 4 years after the last REKAMBYS injection.
Women with HIV should not breastfeed because HIV can be transmitted through breast milk and infect the baby.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Some patients may feel tired, dizzy, or sleepy during treatment with REKAMBYS. Do not drive or use machines if you experience any of these side effects.
Important information about some of the ingredients of REKAMBYS
This medicine contains less than 1 mmol of sodium (23 mg) per 3 ml injection; this is essentially “sodium-free”.
3. How REKAMBYS is administered
A nurse or doctor will give you REKAMBYS as an injection into the muscle of the buttock (intramuscular injection, or IM).
The injection will be given once a month or once every 2 months, along with another injectable medicine called cabotegravir. Your doctor will tell you how often you will receive the medicine.
Before starting treatment with REKAMBYS, your doctor will prescribe a daily treatment with rilpivirine and cabotegravir tablets for a month. This is known as oral lead-in dosing; taking the tablets before receiving the REKAMBYS and cabotegravir injections will allow your doctor to check if these medicines are suitable for you.
If you are going to receive REKAMBYS once a month, your treatment will be as follows:
When | |||
Medicine | Month 1 (at least 28 days) | Month 2 (after 1 month of tablets) | From Month 3 |
Rilpivirine | 25 mg tablet once a day | 900 mg injection | 600 mg injection every month |
Cabotegravir | 30 mg tablet once a day | 600 mg injection | 400 mg injection every month |
If you are going to receive REKAMBYS every 2 months, your treatment will be as follows:
When | |||
Medicine | Month 1 (at least 28 days) | Month 2 (after 1 month of tablets) and Month 3 | From Month 5 |
Rilpivirine | 25 mg tablet once a day | 900 mg injection | 900 mg injection every 2 months |
Cabotegravir | 30 mg tablet once a day | 600 mg injection | 600 mg injection every 2 months |
If you miss an injection of REKAMBYS
It is very important that you regularly attend your scheduled visits to receive your injection. If you do not attend a visit, contact your doctor immediately to schedule another.
Tell your doctorif you think you will not be able to receive the REKAMBYS injection on the scheduled date. Your doctor may recommend that you take tablets until you can receive a REKAMBYS injection again.
If you are given too much REKAMBYS
A doctor or nurse will give you this medicine, so it is unlikely that you will be given too much. If you are concerned, tell your doctor or nurse.
Do not stop using REKAMBYS without your doctor's advice.
Use REKAMBYS for as long as your doctor tells you. Do not interrupt treatment unless your doctor advises you to.
After stopping treatment, low levels of rilpivirine (the active substance of REKAMBYS) may remain in your body for up to 4 years. However, after the last REKAMBYS injection, the low levels of rilpivirine that remain will not be enough to fight the virus, and it may become resistant. To keep your HIV-1 infection under control and prevent the virus from becoming resistant, you must start a different HIV treatment on the date when the next REKAMBYS injection was scheduled.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following is a list of side effects that have been reported with the use of REKAMBYS and cabotegravir injectable.
Very common side effects (affect at least 1 in 10 people)
- headache
- injection site reactions - usually mild to moderate and their frequency decreases over time. Symptoms may include:
o very common: pain and discomfort, lumps or hard masses
o common: redness, itching, swelling, bruising, heat or change in color.
o uncommon: numbness, mild bleeding, abscess formation (collection of pus) or cellulitis (with a feeling of heat, swelling, or redness).
- feeling hot/feverish (pyrexia)
Common side effects (affect less than 1 in 10 people)
- depression
- anxiety
- abnormal dreams
- difficulty sleeping (insomnia)
- dizziness
- feeling sick (nausea)
- vomiting
- stomach pain (abdominal pain)
- gas (flatulence)
- diarrhea
- rash
- muscle pain (myalgia)
- fatigue (fatigue)
- feeling weak (asthenia)
- general malaise
- weight gain
Uncommon side effects (affect less than 1 in 100 people)
- drowsiness (somnolence)
- feeling dizzy during or after an injection. This can cause fainting.
- liver damage (signs may include yellowing of the skin and the white part of the eye, loss of appetite, itching, stomach pain, pale stools, or dark urine).
- changes in liver function test results (increased transaminases)
- increased bilirubin(a substance produced by the liver) in the blood.
Other side effects
- severe stomach pain caused by pancreas inflammation (pancreatitis).
The following side effects that can occur with rilpivirine tablets may also occur with REKAMBYS injectable:
Very common side effects
- increased cholesterol and/or pancreatic amylase in the blood
Common side effects (affect less than 1 in 10 people)
- loss of appetite
- sleep disorders
- depressive state
- stomach upset
- dry mouth
- low white blood cell and/or platelet count, decreased hemoglobin in the blood, increased triglycerides and/or lipase in the blood
Uncommon side effects (affect less than 1 in 100 people)
- signs or symptoms of inflammation or infection, such as fever, chills, sweating (immune reconstitution syndrome, for more information see section 2)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of REKAMBYS
Keep out of sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of REKAMBYS
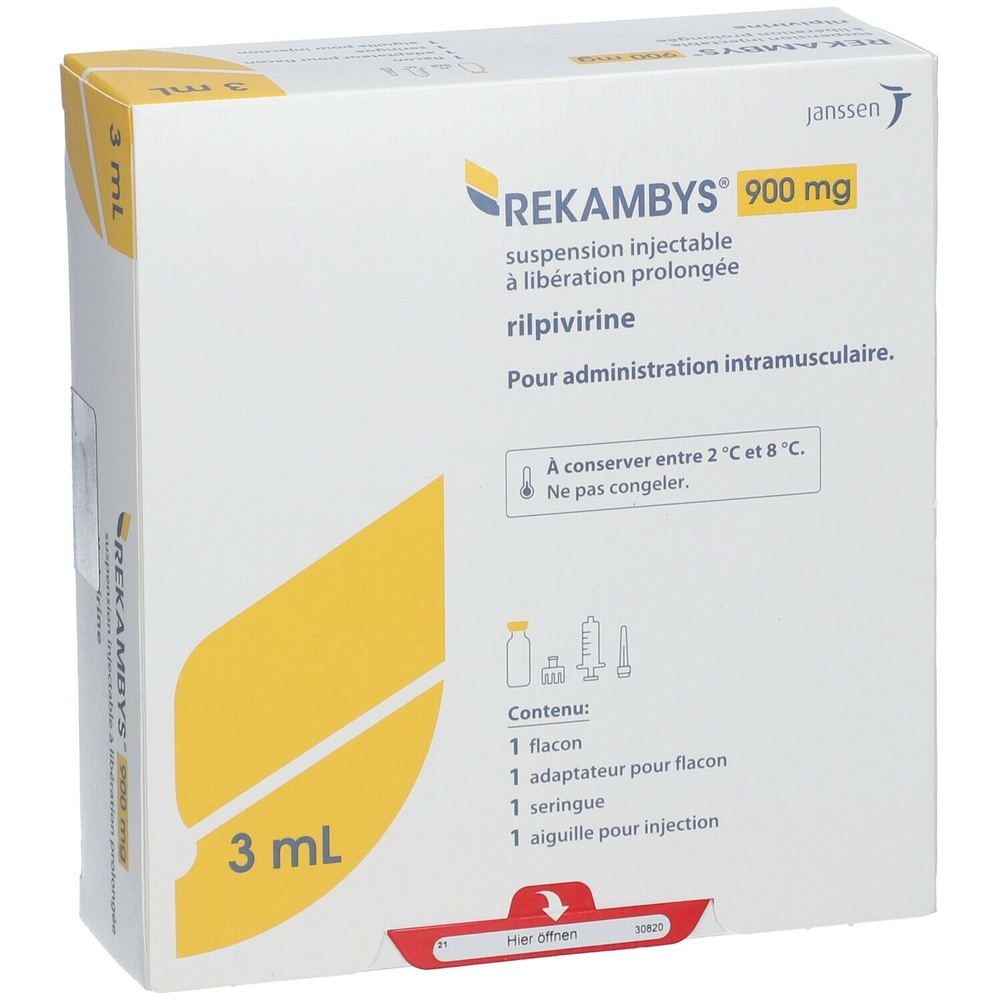
- The active ingredient is rilpivirina. Each 3 ml vial contains 900 mg of rilpivirina.
- The excipients are poloxamer 338, citric acid monohydrate, glucose monohydrate, sodium dihydrogen phosphate monohydrate, sodium hydroxide for pH adjustment and to ensure isotonicity, and water for injectables.
Appearance and Container Contents of the Product
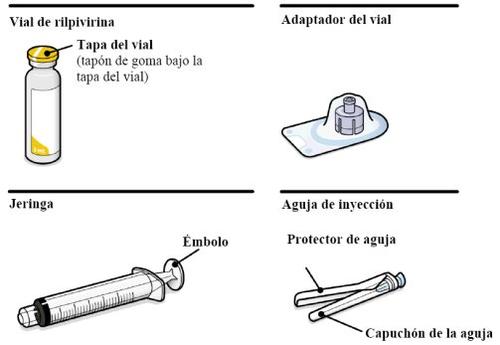
Long-acting injectable suspension. REKAMBYS is presented in a glass vial. The packaging also contains 1 syringe, 1 vial adapter, and 1 injection needle.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien ViiV Healthcare srl/bv Tel: + 32 (0) 10 85 65 00 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
България „Джонсън & Джонсън“ ЕООД Тел.: +359 2 489 94 00 | Luxembourg/Luxemburg ViiV Healthcare srl/bv Belgique/Belgien Tél/Tel: + 32 (0) 10 85 65 00 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD. Tel: +356 2397 6000 |
Deutschland ViiV Healthcare GmbH Tel.: + 49 (0)89 203 0038-10 | Nederland ViiV Healthcare BV Tel: + 31 (0) 33 2081199 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλάδα Janssen-Cilag Φαρμακευτική Α.Ε.Β.Ε. Τηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Laboratorios ViiV Healthcare, S.L. Tel: + 34 900 923 501 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France ViiV Healthcare SAS Tél.: + 33 (0)1 39 17 69 69 | Portugal VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Tel: + 351 21 094 08 01 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson s.r.o. Tel: +421 232 408 400 |
Italia ViiV Healthcare S.r.l Tel: +39 045 7741600 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Κύπρος Βαρνάβας Χατζηπαναγής Λτδ Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom ViiV Healthcare UK Limited Tel: + 44 (0)800 221441 |
Date of the Last Revision of this Leaflet: {MM/AAAA}.
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu/.
This information is intended solely for doctors or healthcare professionals who must read it together with the complete prescribing information (Summary of Product Characteristics).
Instructions for Use of REKAMBYS 3 ml Injectable:
Summary A complete dose consists of two injections: 3 ml of cabotegravir and 3 ml of rilpivirina. Cabotegravir and rilpivirina are presented in suspensions that do not require dilution or reconstitution. The steps for preparing both medications are the same. Cabotegravir and rilpivirina are for intramuscular administration only. Both injections should be administered in the buttock. The order of administration is not important. Note:It is recommended to administer in the ventrogluteal area. | |
Storage Information | |
Do notfreeze. | |
| |
Your Package Contains | |
Take into account the patient's constitution and use your medical judgment to select the appropriate injection needle length. | |
You Will Also Need | |
| |
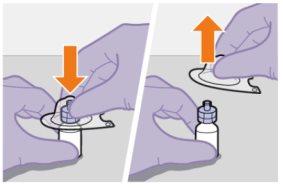
Preparation | |
| |
|
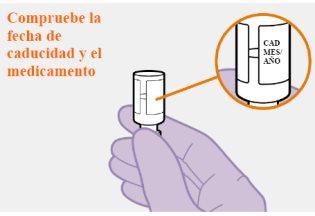
Do not use if the expiration date has passed. |
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
|
| |
|
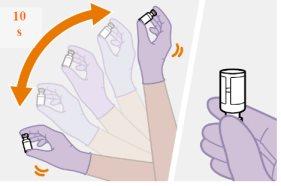
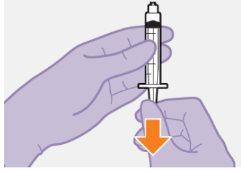
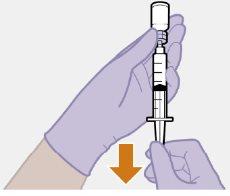
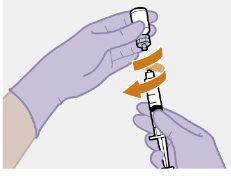
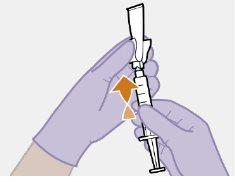
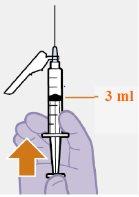
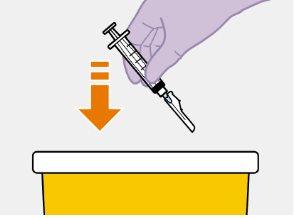
Note: Keep the syringe upright to avoid dripping. Check that the suspension has a uniform appearance and a white milky color. |
| |
|
|
Injection | |
| |
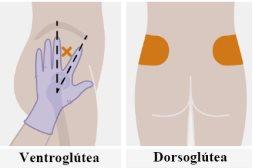
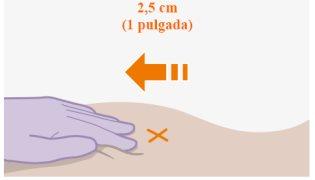
| Injections should be administered in the buttock. Select one of the following areas for injection:
Note: For intramuscular injection in the buttock only. Do notadminister intravenously. |
| |
|
|
| |
|
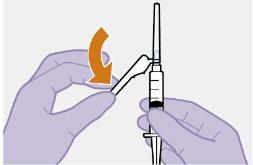
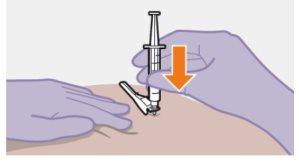
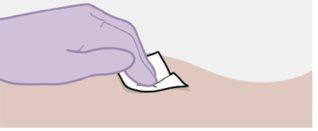
Note: Clean the injection site with an alcohol swab. Allow the skin to dry before proceeding. |
| |
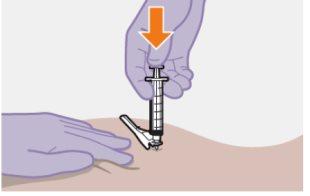
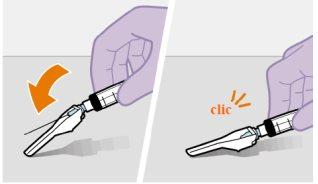
| Use the Z-technique for injection to minimize the medication escaping from the injection site as much as possible.
|
| |
|
|
| |
|
|
| |
|
Do notmassage the area. |
| |
|
|
After the Injection | |
| |
|
|
Repeat the Process for the 2nd Medication | |
| If you have not yet injected both medications, follow the steps for preparing and injecting cabotegravir, which has its own Instructions for Use. |
Questions and Answers | |
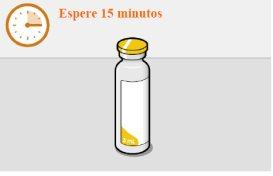
It is best to inject the medication as soon as it reaches room temperature. However, the vial can be left in its packaging at room temperature (maximum temperature of 25°C) for a maximum of 6 hours.
It is best to inject the medication (at room temperature) as soon as possible after it is withdrawn from the vial. However, the medication can be left in the syringe for a maximum of 2 hours before injecting. If more than 2 hours pass, discard the medication, syringe, and needle.
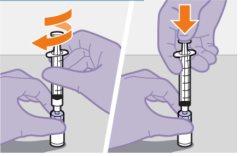
Injecting 1 mL of air into the vial facilitates the withdrawal of the dose with the syringe. If air is not injected, part of the liquid may accidentally return to the vial, leaving insufficient amount in the syringe.
No, the order is not important.
It is best to let the vial reach room temperature naturally. However, you can use the heat from your hands to speed up the process, but make sure the vial does not exceed 25°C Do not use any other method to heat it.
Administration in the ventrogluteal area is recommended because it is an area where there are no major nerves or blood vessels nearby. If the healthcare professional prefers, administration in the dorsogluteal area is also acceptable. The injection should not be administered in any other area. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: TABLET, 25 mg rilpivirineActive substance: rilpivirineManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 600 mgActive substance: efavirenzManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for REKAMBYS 900 MG PROLONGED-RELEASE INJECTABLE SUSPENSION – subject to medical assessment and local rules.