
REANDRON 1000 mg/4 ml INJECTABLE SOLUTION

How to use REANDRON 1000 mg/4 ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Reandron 1000mg / 4ml injectable solution
Testosterone, undecanoate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the pack
- What is Reandron and what is it used for
- What you need to know before you are given Reandron
- How to use Reandron
- Possible side effects
- Storage of Reandron
- Contents of the pack and other information
1. What is Reandron and what is it used for
Reandron contains testosterone, a male hormone, as the active ingredient.
Reandron is given by intramuscular injection; the medicine is released over time.
Reandron is used in adult men for testosterone replacement therapy to treat various health problems caused by a lack of testosterone (male hypogonadism). This should be confirmed by two separate blood testosterone tests and clinical symptoms such as:
- impotence
- infertility
- low sexual desire
- fatigue
- depressive mood
- bone loss caused by low hormone levels
2. What you need to know before you are given Reandron
Do not use Reandron
- if you are allergic to testosterone undecanoate or any of the other ingredients of this medicine (listed in section 6).
- if you have androgen-dependent cancer or suspect you may have prostate or breast cancer.
- if you have or have had a liver tumor;
Reandron is not indicatedfor use in women.
Warnings and precautions
Tell your doctorbefore you start using Reandron if you have or have ever had:
- epilepsy
- heart, liver, or kidney problems
- migraine
- breathing interruptions during sleep (apnea) as it may worsen with treatment
- cancer, as calcium levels in the blood should be determined
- high blood pressure or if you are being treated for high blood pressure, as testosterone may cause an increase in blood pressure.
- bleeding problems
- bleeding disorders (e.g., hemophilia)
- thrombophilia (a blood clotting abnormality that increases the risk of blood clots in blood vessels)
- factors that increase the risk of blood clots in a vein: previous blood clots in a vein, smoking, obesity, cancer, inactivity, if a close relative has had a blood clot in a leg, lung, or other organ at a young age (e.g., below 50 years of age), or as you get older.
How to recognize a blood clot: painful swelling of a leg or sudden change in skin color, for example, becoming pale, red, or blue, sudden difficulty breathing, sudden and unexplained cough that may bring up blood, or sudden chest pain, severe dizziness or fainting, severe stomach pain, sudden loss of vision. Seek urgent medical attention if you experience any of these symptoms.
If you have severe heart, liver, or kidney failure,treatment with Reandron may cause you to have serious complications in the form of fluid retention, which may occasionally be accompanied by congestive heart failure.
Before starting treatment and during treatment, your doctor will check the following parameters in your blood test: testosterone level and complete blood count.
If your liver is not working properly
No formal studies have been conducted in patients with liver failure. If you have ever had a liver tumor, you will not be prescribed Reandron (see "Do not use Reandron").
Children and adolescents
Reandron is notindicatedfor use in children and adolescents. There is no available information on the use of Reandron in males under 18 years of age.
Older people (65 years or older)
No dose adjustment is necessary if you are over 65 years old. (See "Medical examination/Follow-up").
Muscle building and doping tests
Reandron is notindicated for increasing muscle mass in healthy individuals or for increasing physical strength.
Reandron may give positive results in doping tests.
Substance abuse and dependence
Always use this medicine exactly as your doctor or pharmacist has told you.
Abuse of testosterone, especially if you take too much of this medicine alone or in combination with other anabolic androgenic steroids, can cause serious health problems with your heart and blood vessels (which can be fatal), your mental health, and/or your liver.
People who have abused testosterone may become dependent and may experience withdrawal symptoms when the dose is significantly changed or when use is suddenly stopped. Do not abuse this medicine because it can cause serious health problems, whether used alone or in combination with other anabolic androgenic steroids. (see "Possible side effects")
Medical examination / Follow-up
Male hormones can increase the growth of prostate cancer or increase the size of the prostate gland (benign prostatic hyperplasia). Before starting treatment with Reandron, your doctor should perform a medical examination to rule out the risk of existing prostate cancer.
Your doctor should carefully monitor your prostate and breasts, especially in older patients. Your doctor will also perform periodic blood tests.
Benign (non-cancerous) and malignant (cancerous) liver tumors have been reported in patients treated with hormonal products such as androgenic compounds.
Using Reandron with other medicines
Tell your doctor or pharmacistif you are using or have recently used or might use any other medicines, including those obtained without a prescription. Your doctor may need to adjust the dose if you are taking other medicines, such as:
- Adrenocorticotropic hormone (ACTH) or corticosteroids (used to treat various diseases such as rheumatism, arthritis, allergic reactions, and asthma). Reandron may increase the risk of fluid retention, especially if you have heart or liver problems.
- Medicines that make your blood more fluid (oral anticoagulants, coumarin derivatives) as they may increase the risk of bleeding. Your doctor will check your dose.
- Medicines for the treatment of diabetes. It may be necessary to adjust the dose of your antidiabetic medicine. Like other androgens, testosterone may increase the effect of insulin.
Tell your doctor if you have any coagulation disorders,as it is important for your doctor to know this information to decide whether you can be treated with Reandron.
Reandron may also affect the results of laboratory tests (e.g., thyroid gland tests). Inform your doctor or laboratory personnel that you are being treated with Reandron.
Pregnancy and breastfeeding
Reandron is not indicated for use in women and should not be used in pregnant or breastfeeding women.
Fertility
Treatment with high doses of testosterone medicines can interrupt or reduce sperm production in a reversible way (see also "Possible side effects").
Driving and using machines
No effects on the ability to drive or use machines have been observed.
Reandron contains benzyl benzoate
This medicine contains 2000 mg of benzyl benzoate in each 4 ml ampoule/vial, equivalent to 500 mg/ml.
3. How to use Reandron
Your doctor will inject Reandron (1 ampoule/vial) intramuscularly, very slowly. The treatment will be repeated every 10-14 weeks, which is sufficient time to maintain testosterone levels without accumulation in the blood.
Reandron should only be administered intramuscularly. Special care should be taken not to inject the product into a blood vessel (see "Administration").
Starting treatment
Before starting treatment and during the first part of treatment, your doctor will determine your blood testosterone levels. Your doctor may give you a second injection, as early as 6 weeks after the first injection, in order to quickly reach the necessary testosterone level. This will depend on your symptoms and testosterone levels.
Maintaining Reandron levels during treatment
The interval between injections should be maintained within the recommended limit of 10 to 14 weeks. Your doctor will regularly check your testosterone levels at the end of an injection interval to ensure that the levels are correct. If the levels are too low, your doctor may increase the frequency of injections. If the levels are too high, your doctor may decrease the frequency of injections. Remember your treatment days, as otherwise your testosterone levels will not be properly controlled.
If you think the effect of Reandron is too strong or too weak, tell your doctor.
If you use more Reandron than you should
Symptoms of having received too much Reandron include:
- irritability
- nervousness
- weight gain
- frequent or prolonged erections
Tell your doctor if you have any of the above symptoms. Your doctor will reduce the frequency of injections or stop treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effectsare acne and pain at the injection site.
Common side effects(may affect up to 1 in 10 people)
- abnormally high red blood cell counts
- weight gain
- hot flushes
- acne
- prostate enlargement and associated problems
- various reactions at the injection site, such as pain, bruising, or irritation
Uncommon side effects(may affect up to 1 in 100 people)
- allergic reaction
- increased appetite, changes in some blood test results, such as increased sugar or fat
- depression, emotional changes, insomnia, restlessness, aggression, or irritability
- headache, migraine, or tremors
- cardiovascular changes, high blood pressure, or dizziness
- bronchitis, sinusitis, cough, shortness of breath, wheezing, or voice changes
- diarrhea or nausea
- changes in liver test results
- hair loss or various skin reactions (e.g., itching, redness, or dry skin)
- joint or limb pain, muscle problems (e.g., spasms, pain, or stiffness), or increased creatine phosphokinase in the blood
- urinary tract changes (e.g., decreased urine flow, urinary retention, urgent need to urinate at night)
- prostate changes (e.g., prostatic dysplasia, hardening, or inflammation), changes in sexual desire, testicular pain, pain, hardening, or enlargement of the breasts, or increased levels of male and female hormones
- fatigue, generalized weakness, excessive sweating, or night sweats
Rare side effects(may affect up to 1 in 1,000 patients)
- The oily liquid of Reandron may reach the lungs (pulmonary microembolism of oily solutions) and in rare cases may cause signs and symptoms such as cough, shortness of breath, discomfort, excessive sweating, chest pain, dizziness, or fainting. These reactions can occur during or immediately after injection and are reversible.
Suspected anaphylactic reactions have been reported after injection of Reandron.
In addition to those mentioned above, the following side effects have been observed after treatment with testosterone-containing products: nervousness, hostility, brief interruptions of breathing during sleep (apnea), skin reactions such as dandruff and seborrhea, excessive hair growth, more frequent erections, and in very rare cases, yellowing of the skin and eyes (jaundice).
Treatment with high doses of testosterone usually interrupts or reduces sperm production, although it returns to normal after treatment is stopped. Testosterone replacement therapy in cases of low testicular function (hypogonadism) may rarely cause persistent and painful erections (priapism). Long-term or high-dose testosterone treatment may occasionally cause increased fluid retention and edema (swelling due to fluid retention).
In general, with testosterone-based medicines, a frequent risk of increased red blood cell count, hematocrit (percentage of red blood cells in the blood), and hemoglobin (the component of red blood cells that carries oxygen) has been observed, identified by periodic blood tests.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Reandron
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Reandron Composition
The active ingredient is testosterone undecanoate 250 mg/ml (corresponding to 157.9 mg of testosterone). 1 ampoule/vial contains 1,000 mg of testosterone undecanoate (which corresponds to 631.5 mg of testosterone).
The other components are: benzyl benzoate and refined castor oil.
Product Appearance and Container Content
Reandron is a clear oily solution, between colorless and light yellowish-brown. It is presented in topaz-colored glass ampoules/vials, which contain 4 ml of injectable solution.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder Grünenthal Pharma, S.A. Doctor Zamenhof, 36 28027 Madrid Spain | Manufacturer Bayer AG 13342 Berlin Germany or EVER Pharma Jena GmbH Brüsseler Strasse 18 07747 Jena Germany or Grünenthal GmbH Zieglerstrasse 6 52078 Aachen Germany or Grünenthal Pharmaceuticals GmbH & Co. KG Philipp-Ott-Straße 3 51373 Leverkusen Germany |
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
- Cyprus, Czech Republic, Greece, Denmark, Luxembourg, Malta, Poland, and Portugal: Nebido
- Austria: Nebido 1000 mg/4 ml Injektionslösung
- Belgium: Nebido 1000 mg/4 ml, oplossing voor injectie
- Bulgaria: ?????? 250 mg/ml ??????????? ???????
- Croatia: Nebido 1000 mg/4 ml otopina za injekcij
- Finland: Nebido 1000 mg/4 ml injektioneste, liuos
- France: Nebido 1000 mg/4 ml, solution injectable
- Germany: Nebido 1000 mg Injektionslösung
- Hungary: Nebido 250 mg/ml oldatos injekció
- Iceland: Nebido 1000 mg/4 ml stungulyf, lausn
- Italy: NEBIDO 1000 mg/4ml soluzione iniettabile
- Lithuania: Nebido 1000 mg/4 ml injekcinis tirpalas
- Netherlands: Nebido 1000 mg/4 ml
- Norway: Nebido 1000 mg/4 ml injeksjonsvæske, oppløsning
- Romania: Nebido 1000 mg/ 4 ml solutie injectabila
- Slovakia: Nebido 1000 mg/4 ml injekcný roztok
- Slovenia: Nebido 1000 mg/4 ml raztopina za injiciranje
- Spain: Reandron 1000 mg/4 ml solución inyectable
- Sweden: Nebido, 1000 mg/4 ml injektionsvätska, lösning
- United Kingdom and Ireland: Nebido 1000mg/4ml, solution for injection
Date of the last revision of this leaflet:October 2022
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
At cold storage temperatures, the properties of this oily solution may change temporarily (e.g., increased viscosity, turbidity). If stored at cold temperatures, the product should be brought to room temperature or body temperature before use.
The solution for intramuscular injection should be inspected visually before use and only clear solutions free of particles should be used.
The contents of an ampoule/vial should be injected intramuscularly immediately after opening the ampoule/vial.
The medicinal product is for single use only, and any unused solution should be discarded.
Administration
Great care should be taken not to inject the medicinal product into a blood vessel.
As with all oily solutions, Reandron should be injected only intramuscularly and very slowly. Pulmonary microembolism related to oily solutions can rarely cause signs and symptoms such as cough, dyspnea, discomfort, hyperhidrosis, chest pain, dizziness, paresthesia, or syncope. These reactions can occur during or immediately after injection and are reversible. Treatment is usually supportive, e.g., administration of supplemental oxygen.
Suspected anaphylactic reactions have been reported after administration of Reandron.
Warnings
A careful regular check of the prostate and breasts should be performed according to established methods (digital rectal examination and PSA determination in serum) in patients receiving testosterone treatment, at least once a year and twice a year in elderly patients and those at risk (those with clinical or family factors).
In addition to laboratory tests to determine testosterone concentration in blood in patients undergoing long-term treatment, the following laboratory tests should be performed periodically: hemoglobin, hematocrit, liver function tests, and lipid profile.
In patients with severe heart, liver, or kidney failure or with ischemic heart disease, treatment with testosterone may cause serious complications, characterized by edema with or without congestive heart failure. In this case, treatment should be discontinued immediately.
Instructions for Opening Reandron Ampoule with "One-Point Cut" (UPC) Opening System
The ampoule has a mark below the colored point. Before opening, ensure that no solution remains in the top part of the ampoule. Use both hands to open it; hold the lower part of the ampoule with one hand and use the other hand to press outward and break the top part of the ampoule in the direction opposite the colored point.

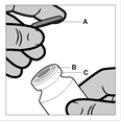
Instructions for Opening Reandron Vial
The vial is for single use. The contents of the vial should be injected intramuscularly immediately after loading into the syringe. After removing the plastic cap (A), do not remove the metal ring (B) or the rim cap (C).
- Country of registration
- Average pharmacy price89.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REANDRON 1000 mg/4 ml INJECTABLE SOLUTIONDosage form: GEL, 2% testosteroneActive substance: testosteroneManufacturer: Advanz Pharma LimitedPrescription requiredDosage form: GEL, 23 mg/gActive substance: testosteroneManufacturer: The Simple Pharma Company LimitedPrescription requiredDosage form: INJECTABLE, Testosterone Propionate 25 mg/mlActive substance: testosteroneManufacturer: Desma Laboratorio Farmaceutico S.L.Prescription required
Online doctors for REANDRON 1000 mg/4 ml INJECTABLE SOLUTION
Discuss questions about REANDRON 1000 mg/4 ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












