
RAVICTI 1.1 G/ML ORAL LIQUID


How to use RAVICTI 1.1 G/ML ORAL LIQUID
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Ravicti1,1g/ml oral liquid
glycerol phenylbutyrate
Read this entire summary of product characteristics carefully before you start taking this medicine, as it contains important information for you.
- Keep this summary of product characteristics, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this summary of product characteristics. See section 4.
Contents of the Summary of Product Characteristics
- What is Ravicti and what is it used for
- What you need to know before you take Ravicti
- How to take Ravicti
- Possible side effects
- Storage of Ravicti
- Package contents and further information
1. What is RAVICTI and what is it used for
Ravicti contains the active substance glycerol phenylbutyrate, which is used to treat six known urea cycle disorders (UCDs) in adults, adolescents, and children. UCDs include deficiencies of certain liver enzymes, such as carbamoyl phosphate synthetase I (CPS), ornithine transcarbamylase (OTC), argininosuccinate synthetase (ASS), argininosuccinate lyase (ASL), arginase I (ARG), and hyperammonemia-hyperornithinemia-homocitrullinuria syndrome (HHH).
RAVICTI should be combined with a low-protein diet and, in some cases, with a diet supplemented with essential amino acids (such as arginine, citrulline, and non-protein calorie supplements).
About Urea Cycle Disorders
- In urea cycle disorders, the body cannot remove nitrogen from the proteins we consume.
- Normally, the body converts excess nitrogen from proteins into a waste product called ammonia. The liver then removes ammonia from the body through a cycle called the urea cycle.
- In urea cycle disorders, the body is unable to produce sufficient liver enzymes to remove excess nitrogen.
- As a result, ammonia accumulates in the body. If ammonia is not removed from the body, it can damage the brain and cause partial loss of consciousness or coma.
- Urea cycle disorders are rare.
How RAVICTI works
Ravicti helps the body remove waste nitrogen. This reduces the amount of ammonia in the body.
2. What you need to know before you take RAVICTI
Do not takeRavicti
- if you are allergic to glycerol phenylbutyrate.
- if you have hyperammonemia (high levels of ammonia in the blood) that requires more rapid intervention (see the section "Warnings and precautions").
If you are unsure whether you are in any of the above situations, consult your doctor or pharmacist before taking Ravicti.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Ravicti:
- if you have liver or kidney problems, as RAVICTI is eliminated from the body through these organs.
- if you have pancreas, stomach, or intestine problems, as these organs are responsible for the absorption of RAVICTI in the body.
If you are in any of the above situations, consult your doctor or pharmacist before taking Ravicti.
In some cases, such as infection or post-operative, the amount of ammonia may increase despite treatment with glycerol phenylbutyrate and may damage the brain (hyperammonemic encephalopathy).
In other cases, the amount of ammonia in the blood may increase rapidly. In this case, RAVICTI will not cause the amount of ammonia in the blood to increase to excessively high levels.
High levels of ammonia in the blood cause nausea, vomiting, or a feeling of confusion.
Tell your doctor or go to the hospital immediately if you notice any of these signs.
It will be necessary to perform blood tests so that your doctor can determine and maintain the correct dose for you.
Other medicines and RAVICTI
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines, which may be less effective when used with RAVICTI. If you are taking these medicines, you may need to have regular blood tests:
- midazolam and barbiturates (used for sedation, sleep problems, or epilepsy)
- contraceptives
Also, tell your doctor if you are taking any of the following medicines, as they may increase the amount of ammonia in the body or change the way RAVICTI works:
- corticosteroids (used to treat inflamed areas of the body)
- valproic acid (a medicine for epilepsy)
- haloperidol (used to treat some mental health problems)
- probenecid (for treating high levels of uric acid in the blood, which can cause gout ["hyperuricemia"])
- lipase inhibitors (such as orlistat): used to treat obesity
- lipase in pancreatic enzyme replacement treatments.
If you are in any of the above situations (or are unsure), consult your doctor before taking Ravicti.
Pregnancy, contraception, and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using Ravicti. If you become pregnant during treatment with Ravicti, consult your doctor. RAVICTI should not be used during pregnancy, as the risks to the fetus cannot be ruled out.
- If you are a woman who can become pregnant, you must use an effective contraceptive method during treatment with Ravicti. Consult your doctor about which contraceptive method is best for you.
- If you plan to breastfeed while taking RAVICTI, you should talk to your doctor. It will be necessary to decide whether to stop breastfeeding or stop treatment, considering the benefit of breastfeeding for the child and the benefit of treatment for you, as RAVICTI may pass into breast milk and the risk to newborns/children cannot be excluded.
Driving and using machines
Ravicti's influence on the ability to drive and use machines is important. When you take RAVICTI, you may feel dizzy or have a headache. Do not drive or use machines while you are experiencing these side effects.
3. How to take RAVICTI
Follow the instructions for administering this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
You must follow a special low-protein diet during treatment with RAVICTI.
- This diet will be designed for you by your doctor and dietitian.
- You must follow it carefully.
- You may need to take amino acid supplements.
- You will need to receive treatment and follow a diet for the rest of your life, unless you have a successful liver transplant.
Amount to be taken
Your doctor will tell you how much Ravicti to take each day.
- Your daily dose will depend on your size and weight, the amount of protein in your diet, and the overall condition of the urea cycle disorder.
- Your doctor may give you a lower dose if you have kidney or liver problems.
- You will need regular blood tests so that your doctor can determine the correct dose for you.
- Your doctor may tell you to take Ravicti more than 3 times a day. In young children, it may be 4 to 6 times a day. There should be at least 3 hours between each dose.
Taking this medicine
Your doctor will tell you how to take Ravicti oral liquid. It can be taken in the following ways:
- by mouth
- through a tube that goes from the abdomen to the stomach, called a "gastrostomy tube"
- through a tube that goes from the nose to the stomach, called a "nasogastric tube".
Take RAVICTI by mouth unless your doctor tells you otherwise.
Ravicti and meals
Take Ravicti during or just after a meal. Young children should receive the medicine during or just after feeding.
Dose measurement
- Use an oral syringe to measure your dose.
- You must have the RAVICTI bottle with an oral syringe to administer the correct amount of RAVICTI.
- Open the RAVICTI bottle by pressing the cap and turning it to the left.
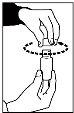
- Place the end of the oral syringe into the syringe insert integrated into the bottle.
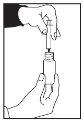
- Hold the bottle upside down while keeping the oral syringe inserted.
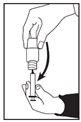
- Fill the oral syringe by pulling the plunger back until the syringe is filled with the amount of RAVICTI liquid that your doctor has told you to take.
- Note: If possible, use the oral syringe size in milliliters that is closest to the recommended dose, without being less than this dose (for example, if the dose is 0.8 ml, use a 1 ml oral syringe).
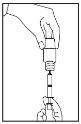
- Tap the oral syringe to remove air bubbles, making sure you have filled it with the correct amount of liquid.
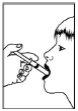
- Swallow the liquid from the oral syringe or connect the oral syringe to a gastrostomy tube or nasogastric tube.
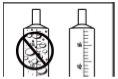
- Important note:Do not add Ravicti to large volumes of liquid or mix it with these, as RAVICTI is heavier than most liquids. If you mix RAVICTI with large volumes of liquid, you may not get the full dose.
- RAVICTI can be added to a small amount of soft foods, such as ketchup, medical formulas, apple sauce, or pumpkin puree.
- If the volume of the oral syringe is less than the prescribed dose, you will need to repeat these steps to get the full dose. Use an oral syringe for all doses taken each day.
- After taking the full dose, drink a little water to make sure no medicine is left in the mouth, or flush the nasogastric or gastrostomy tube with 10 ml of water using a new oral syringe. The syringe used to flush the nasogastric or gastrostomy tube must not be used to measure the dose of RAVICTI, to avoid contact between water and the medicine.

- Close the bottle by screwing the cap.
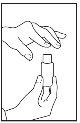
- Important note:Do not rinse the oral syringe between daily doses, as adding water causes RAVICTI to degrade. If RAVICTI comes into contact with water, the liquid will appear cloudy. Keep the bottle and oral syringe in a clean and dry place between doses.
- Discard the oral syringe after the last dose of the day. Do not reuse the oral syringe to measure the dose of RAVICTI on another day.
- Unused syringes should be kept to use with another bottle. Each bottle must be discarded after 14 days.
If you take more RAVICTI than you should
If you take more of this medicine than you should, talk to your doctor.
If you notice any of the following symptoms, talk to a doctor or go to a hospital immediately, as they may be signs of overdose or high ammonia:
- feeling sleepy, tired, or dizzy, sometimes confused
- headache
- changes in taste
- hearing problems
- feeling disoriented
- reduced ability to remember things
- existing neurological disorders may worsen.
If you forget to take RAVICTI
If you forget a dose, take it as soon as you remember. However, in adults, if the next dose is due in less than 2 hours, skip the missed dose and take the next dose at the usual time.
For children: if the next dose is due in less than 30 minutes, skip the missed dose and give the next dose at the usual time.
- Do not take a double dose to make up for missed doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
If you stop taking RAVICTI
You will need to take this medicine and follow a special low-protein diet for the rest of your life. Do not stop taking RAVICTI without talking to your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any side effects, talk to your doctor or pharmacist.
This medicine can cause the following side effects:
Common:may affect up to 1 in 10 people
- swelling or stomach pain, constipation, diarrhea, stomach acid, gas, vomiting, feeling sick (nausea), mouth pain, retching
- swelling of the hands or feet, feeling tired
- feeling dizzy, headache, or shaking
- decreased or increased appetite
- aversion to certain foods
- bleeding between menstrual periods
- acne, unusual skin odor
- blood test results showing increased liver enzymes, electrolyte imbalance, low white blood cell count (lymphocytes), or low vitamin D levels.
Uncommon:may affect up to 1 in 100 people
- dry mouth
- belching, stomach pain or discomfort, changes in stool, such as oily appearance, urgent need to defecate, painful defecation, inflammation of the mouth and lips
- feeling hungry
- increased temperature
- hot flashes
- gallbladder pain
- bladder pain
- back pain, joint pain, muscle spasms, pain in the arms or legs, heel spur syndrome
- gastrointestinal viral infection
- tingling sensation, feeling very restless, drowsiness, speech problems, feeling confused, feeling depressed, altered taste
- cessation of menstruation or irregular menstrual periods
- voice changes, nosebleeds, nasal congestion, or throat inflammation or pain
- hair loss, increased sweating, itchy skin rash
- irregular heartbeat
- decreased thyroid function
- weight loss or gain
- blood test results showing increased or decreased potassium levels in the blood
- blood test results showing increased levels of triglycerides, low-density lipoproteins, or white blood cells in the blood
- abnormal electrocardiogram (ECG) results
- increased prothrombin time in blood tests
- blood test results showing decreased albumin levels in the blood
Side effects in children under 2 months
The following side effects have been observed in a clinical study with 16 patients under 2 months of age:
- diarrhea, constipation, flatulence, reflux of stomach contents, insufficient feeding
- rash
- low red blood cell count
- high platelet count (which can cause blood to clot)
- increased liver enzymes
- decreased amino acid levels
Side effects in children from 2 months to less than 2 years of age
- diarrhea, constipation
- eczema, nail striations, rash
Reporting side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this summary of product characteristics. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of RAVICTI
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and bottle label, after EXP. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions. Once the bottle is opened, you must use this medicine within 14 days of opening. The bottle should be discarded even if it is not empty.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Packaging Contents and Additional Information
Composition ofRavicti
- The active ingredient is glycerol phenylbutyrate.
- Each milliliter of the liquid contains 1.1 g of glycerol phenylbutyrate. This corresponds to a density of 1.1 g/ml.
- There are no other components.
Appearance of the Product andPackaging Contents
The liquid is contained in a 25 ml transparent glass bottle with a child-resistant plastic cap.
To ensure the correct administration of RAVICTI, oral syringes with CE marking, with a suitable size for the dose and compatible with the syringe insertion piece, can be obtained from the pharmacy. Ask your doctor or pharmacist what type of syringes you need, according to the prescribed dose volume.
Marketing Authorization Holderand Manufacturer
Marketing Authorization Holder
Immedica Pharma AB
SE-113 63 Stockholm
Sweden
Manufacturer
Unimedic AB
Storjordenvägen 2
SE-864 31 Matfors
Sweden
Date of the Last Revision of this Leaflet: 10/2022
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RAVICTI 1.1 G/ML ORAL LIQUIDDosage form: TABLET, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: CAPSULE, 84 mg of eliglustat (as tartrate)Active substance: eliglustatManufacturer: Sanofi B.V.Prescription required
Online doctors for RAVICTI 1.1 G/ML ORAL LIQUID
Discuss questions about RAVICTI 1.1 G/ML ORAL LIQUID, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















