PROTHROMPLEX TOTAL 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use PROTHROMPLEX TOTAL 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Prothromplex Total 500 UI
Powder and Solvent for Solution for Injection
human prothrombin complex
Read all of this leaflet carefully before you start usingthis medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Prothromplex Total and what is it used for
- What you need to know before you start using Prothromplex Total
- How to use Prothromplex Total
- Possible side effects
- Storage of Prothromplex Total
- Contents of the pack and other information
1. What is Prothromplex Total and what is it used for
Prothromplex Total is prepared from human plasma (the liquid part of the blood). It contains blood clotting factors II, VII, IX, and X (prothrombin complex clotting factors) and protein C.
These clotting factors are dependent on vitamin K and, like vitamin K, play an important role in blood clotting. In the event of a deficiency of any of these factors, the blood does not clot as quickly as usual, which increases the tendency to bleed.
Prothromplex Total is used for:
- treatment of bleeding
- prevention of bleeding immediately before or after surgical operations.
- acquired and congenital deficiency of clotting factors
Acquired deficiency:
You may develop a deficiency of vitamin K-dependent clotting factors (acquired deficiency) caused, for example, by treatment or overdose of medications that reduce the effect of vitamin K (known as vitamin K antagonists).
Congenital deficiency:
If you are born with a deficiency (congenital deficiency), this medicine may be administered immediately before or after a surgical operation when the appropriate individual factor concentrate is not available.
2. What you need to know before you start using Prothromplex Total
Do not use Prothromplex Total
- if you are allergic to clotting factors or to any of the other components of this medicine (listed in section 6).
- if heparin has caused or is suspected to have caused a decrease in platelets, the cells responsible for blood clotting (heparin-induced thrombocytopenia).
Warnings and precautions
Consult your doctor before using Prothromplex Total
- since there is a rare possibility that you may develop a severe, sudden allergic reaction (anaphylactic reaction) to Prothromplex Total because such allergic reactions have been reported.
You can find detailed information on the first symptoms of this type of allergic reaction in section 4 "Possible side effects"
- if you have an acquired deficiency of vitamin K-dependent clotting factors.
This acquired deficiency may be caused by treatment with medications that neutralize blood clotting through vitamin K inhibition. In this case, Prothromplex Total should only be used when rapid correction of prothrombin complex levels is necessary, such as in cases of severe bleeding or emergency surgery. In other cases, it is sufficient to reduce the dose of the vitamin K antagonist or administer vitamin K
- if you are taking medications to inhibit blood clotting (vitamin K antagonists). You may have a tendency to form blood clots that can be increased by the administration of human prothrombin complex concentrate
- if you have a congenital deficiencyof specific clotting factors dependent on vitamin K, a product with the specific factor should be used if available
- if you are being treated with human prothrombin complex concentrate, especially if you have received it repeatedly, as blood clots (thrombosis) and embolism may occur
- if you belong to any of the following patient groups, due to the possibility of blood clots:
- patients with coronary heart disease or who have had a heart attack
- patients with liver disease
- pre- or post-operative patients
- newborns
- patients at risk of thromboembolic complications or disseminated intravascular coagulation (DIC)
In all these situations, your doctor will carefully evaluate the benefits of treatment with Prothromplex Total against the possible risks of these complications.
Traceability
Each time you are administered a dose of Prothromplex Total, it is recommended to record the name and batch number of the product to maintain control of the batches used.
Viral safety
When administering medicines derived from human plasma or blood, certain measures must be taken to prevent infections from being transmitted to patients.
Such measures include:
- careful selection of donors to exclude those at risk of being carriers of infectious diseases,
- analysis of specific infection markers in individual donations and plasma pools,
- inclusion of stages in the manufacturing process to eliminate/inactivate viruses.
Despite this, when administering medicines derived from human blood or plasma, the possibility of transmitting infectious agents cannot be entirely excluded. This also applies to emerging or unknown viruses or other types of infections.
These measures are considered effective against enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and against the non-enveloped hepatitis A virus. The measures taken may have limited value against other non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for individuals with a depressed immune system or patients with certain types of anemia (e.g., sickle cell disease or hemolytic anemia).
It is possible that your doctor may recommend vaccination against hepatitis A and hepatitis B if you are regularly or repeatedly administered human prothrombin complex concentrates derived from human plasma.
It is strongly recommended that, each time a dose of Prothromplex Total is administered, a record be kept of the name of the medicine and batch number administered in order to maintain a record of the batches used.
Children and adolescents
The safety and efficacy of using Prothromplex Total in patients under 18 years of age have not been established in clinical trials.
Other medicines and Prothromplex Total
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Tell your doctor if you are taking medications to inhibit blood clotting (vitamin K antagonists). You may have a tendency to form blood clots that can be increased by the administration of human prothrombin complex concentrate.
Interference with biological tests
When performing heparin-sensitive coagulation tests in patients receiving high doses of human prothrombin complex, the heparin contained in the administered product should be considered.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Prothromplex Total can be used during pregnancy and breastfeeding only in cases where it is clinically indicated.
No information is available on the effects of Prothromplex Total on fertility.
Driving and using machines
No studies have been performed on the ability to drive and use machines.
Prothromplex Total contains sodium and heparin
This medicine contains 68 mg of sodium per vial or 0.14 mg of sodium (main component of cooking/table salt) per International Unit. This is equivalent to 3.4% of the maximum recommended daily sodium intake for an adult.
Heparin can cause allergic reactions and a reduction in blood cell count that can affect the blood clotting system. Patients with a history of heparin-induced allergic reactions should avoid using medicines that contain heparin.
3. How to use Prothromplex Total
The start, administration, and monitoring of treatment should be performed under the strict supervision of a doctor experienced in the treatment of coagulation disorders.
The dose and duration of treatment with Prothromplex Total will depend on several factors such as body weight, severity of the disease, location, and intensity of bleeding or the need to prevent bleeding in surgical procedures.
Your doctor will calculate the dose according to your specific needs and regularly monitor coagulation and your clinical condition (see section "This information is intended only for healthcare professionals").
Method of administration
Intravenous route.
A doctor should supervise the administration of Prothromplex Total.
After reconstitution with the provided sterile water for injection, Prothromplex Total is administered slowly into a vein (intravenously). The rate of administration depends on your well-being and should not exceed 2 ml per minute (60 IU/min).
Use in children and adolescents
There are insufficient data to recommend the administration of Prothromplex Total in patients under 18 years of age.
If you use more Prothromplex Total than you should
In case of overdose, the risk of thromboembolic complications or consumption coagulopathy increases.
When high doses of human prothrombin complex concentrates have been administered, myocardial infarction, increased consumption of platelets and clotting factors with elevated clot formation in blood vessels (DIC, consumption coagulopathy), venous thrombosis, and pulmonary embolism have been observed.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
As with all plasma-derived treatments, there is a possibility that you may develop a sudden allergic reaction (anaphylactic reaction). In individual cases, this can range from a severe hypersensitivity reaction to anaphylactic shock.
Therefore, you should be aware of the possible early symptoms of an allergic reaction, such as:
- erythema (redness of the skin)
- rash
- appearance of hives on the skin (urticaria)
- itching all over the body
- swelling of the lips and tongue
- difficulty breathing/dyspnea
- chest tightness
- general malaise
- dizziness
- drop in blood pressure
If you notice one or more of these symptoms, inform your doctor immediately, who will stop the infusion immediately. Severe symptoms require immediate emergency treatment.
When using human prothrombin complex concentrates (including Prothromplex Total), patients may develop resistance (inhibitors) to one or more clotting factors, resulting in the inactivation of blood clotting factors. The appearance of these inhibitors may manifest as an insufficient response to treatment.
During treatment with human prothrombin complex concentrates, blood clots (thrombi) and embolism may occur. This can lead to complications such as myocardial infarction, increased consumption of platelets and clotting factors with elevated clot formation in blood vessels (consumption coagulopathy), venous thrombosis, and pulmonary embolism.
The following side effects may affect up to 1 in 10 people using Prothromplex Total:
- formation of blood clots throughout the body (disseminated intravascular coagulation), resistance (inhibitors) to one or more of the prothrombin complex factors (factors II, VII, IX, X)
- severe, sudden allergic reaction (anaphylactic shock), anaphylactic reaction, hypersensitivity, stroke, headache
- myocardial infarction (acute myocardial infarction), heart palpitations (tachycardia)
- arterial thrombosis, venous thrombosis, drop in blood pressure (hypotension), redness of the skin (flushing)
- blockage of a pulmonary blood vessel by a blood clot (pulmonary embolism),difficulty breathing, shortness of breath (dyspnea), wheezing
- vomiting, feeling of vomiting (nausea)
- rash all over the body (urticaria), skin rash (erythematous rash), itching (pruritus)
- a certain kidney disorder with symptoms such as swelling of the eyelids, face, and lower legs with weight gain, as well as loss of protein through the urine (nephrotic syndrome)
- fever (pyrexia).
The following side effects have been observed with other human prothrombin complex concentrates:
- swelling of the face, tongue, and lips (angioedema), skin sensation like burning, tingling, itching, or numbness (paresthesia)
- reaction at the injection site
- lethargy
- restlessness
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Prothromplex Total
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the medicine in the original packaging to protect it from light.
Keep this medicine out of the sight and reach of children.
Do not use the medicine after the expiry date that appears on the packaging after EXP. The expiry date is the last day of the month indicated.
During the validity period, the product can be stored at room temperature (max. 25°C) for a period of up to 6 months. The start and end of storage at room temperature should be noted on the medicine packaging.
After storage at room temperature, Prothromplex Total should not be returned to the refrigerator (2°C to 8°C) and should be discarded if not used within 6 months.
Use the solution immediately after reconstitution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Prothromplex Total
Powder:
The active ingredient is the human prothrombin complex, composed of human coagulation factors II, VII, IX, and X, and protein C.
Per Vial [UI] | After Reconstitution with 17 ml of Sterile Water for Injectable Preparations [UI/ml] | |
Human Coagulation Factor II | 375 – 708 | 22.5 – 42.5 |
Human Coagulation Factor VII | 417 | 25 |
Human Coagulation Factor IX | 500 | 30 |
Human Coagulation Factor X | 500 | 30 |
A vial contains at least 333 UI of co-purified protein C with the blood coagulation factors.
The other components are: sodium chloride, sodium citrate, sodium heparin (0.2-0.5 UI/UI of factor IX), and antithrombin III 12.5-25 UI per vial (0.75-1.5 UI/ml).
Solvent: Sterile water for injectable preparations.
Appearanceof the Productand Container Content
Powder and solvent for injectable solution.
Prothromplex Total is a white or slightly yellow powder, lyophilized or in the form of a compact dry substance.
After reconstitution, the solution has a pH between 6.5 and 7.5 and an osmolality of not less than 240 mosm/kg. The solution is transparent or slightly opalescent.
The powder and solvent are contained in single-dose glass vials (Type I and Type II hydrolytic glass, respectively) and sealed with butyl rubber stoppers.
Container Content
- 1 vial with Prothromplex Total 500 UI powder
- 1 vial with 17 ml of sterile water for injectable preparations
- 1 Mix2vial for reconstitution
Container Size1 x 500 UI
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Vienna
Austria
Manufacturer:
Takeda Manufacturing Austria AG
Industriestrasse, 67
A-1221 Vienna, Austria
Local Representative:
Takeda Farmacéutica España S.A.
Calle Albacete, 5, 9th floor
Edificio Los Cubos
28027 Madrid
Spain
Tel: +34 91 790 42 22
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria | Prothromplex TOTAL 500 I.E. Powder and solvent for the preparation of an injectable solution |
Estonia, Greece, Hungary, Ireland, Latvia, Malta, Romania, United Kingdom (Northern Ireland) | Prothromplex Total |
Bulgaria, Czech Republic, Poland | Prothromplex Total NF |
Belgium, Denmark, Lithuania, Luxembourg, Netherlands, Norway, Portugal | Prothromplex |
Germany, Slovakia | Prothromplex NF |
Italy, Slovenia | Proplex |
Spain | Prothromplex Total 500 UI powder and solvent for injectable solution |
Dateof the Last Revision of this Leaflet:March 2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------
This information is intended solely for healthcare professionals:
Instructions for Preparation and Administration
General Instructions
- For reconstitution, use only the reconstitution equipment provided.
- Check the expiration date and ensure that Prothromplex Total powder and water for injectables (solvent) are at room temperature before preparation. Do not use after the expiration date indicated on the labels and container.
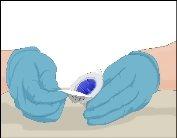
- Use an aseptic technique (clean and low-germ conditions) and a flat work surface during the reconstitution procedure. Wash your hands and put on clean examination gloves (glove use is optional).
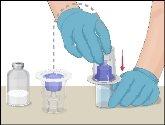
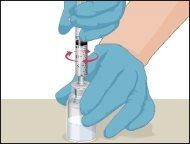
- Warm the unopened vial containing the solvent (sterile water for injections) to room temperature or body temperature (maximum 37°C).
- Prothromplex Total should only be reconstituted immediately before administration. The solution is transparent or slightly opalescent. Do not use cloudy solutions or those containing deposits.
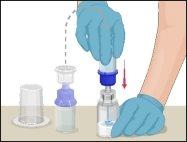
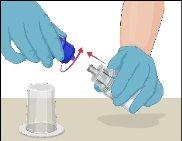
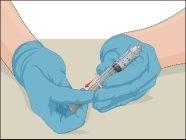
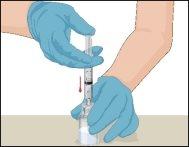
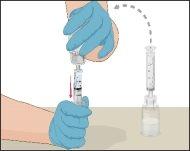
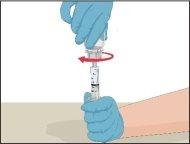
Instructions for Reconstitution of Powder for Injectable Solution:
Steps | Image | |
1 |
|
|
2 |
|
|
3 |
|
|
4 |
|
|
5 |
|
|
6 |
|
|
7 |
|
|
8 |
|
|
9 |
|
|
10 |
|
|
11 |
|
|
12 |
|
|
Administration Instructions
Examine the prepared solution in the syringe for particles and color changes before administration. The solution should be transparent, colorless, and particle-free. The filter included in the Mix2Vial device completely removes these particles. Filtration does not influence dose calculation. The solution in the syringeshould not be used if it is cloudy or contains flakes or particles after filtration.
- Connect the infusion needle to a syringe containing Prothromplex Total solution. For greater convenience, a winged infusion set (butterfly) is recommended. Point the needle upwards and remove any air bubbles by gently tapping the syringe with your finger and slowly pushing the air out of the syringe and needle.
- Apply a tourniquet and prepare the infusion site by thoroughly cleaning the skin with a sterile swab soaked in alcohol (or another suitable sterile solution).
- Insert the needle into the vein and remove the tourniquet. Infuse Prothromplex Total slowly. Do not infuse more than 2 ml per minute. Disconnect the empty syringe.
Note:Do not remove the butterfly needle until all syringes have been infused and do not touch the Luer port that connects to the syringe.
- Remove the needle from the vein and use a sterile swab to apply pressure to the infusion site for several minutes.
Do not recap the needle. Place the needle, syringe, and empty Prothromplex Total and solvent vials in a rigid-walled puncture-proof container for proper disposal. Do not discard these supplies in ordinary household waste.
------------------------------------------------------------------------------------------------------------------
Dosage and Administration
Dosage
The following are only general dosage guidelines, except for the treatment of hemorrhages and perioperative prophylaxis of bleeding during treatment with vitamin K antagonists.
Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders. The dose and duration of replacement therapy depend on the severity of the disorder, the location and intensity of the hemorrhage, and the patient's clinical condition.
The amount and frequency of administration should be calculated individually for each patient. The dosing intervals should be adapted to the different circulating half-lives of the various coagulation factors in the prothrombin complex.
Individual dosage requirements can only be identified based on periodic determination of plasma levels of the relevant coagulation factors or global analysis of prothrombin complex levels (e.g., Quick test, INR, prothrombin time) and continuous monitoring of the patient's clinical condition.
In the case of major surgical interventions, it is essential to perform precise monitoring of replacement therapy through coagulation analysis (specific assays for specific coagulation factors and/or global assays to measure prothrombin complex levels).
Hemorrhage and Perioperative Prophylaxis of Bleeding during Treatment with Vitamin K Antagonists:
In severe hemorrhages or before undergoing operations with a high risk of bleeding, normal levels should be achieved (Quick test 100%, INR 1.0).
The following rule applies: 1 UI of factor IX per kg of body weight increases the Quick test value by approximately 1%.
If administration of Prothromplex Total is based on INR values, the dose will depend on the INR value before treatment and the INR value to be achieved.
The doses described in the following table should be followed according to the recommendations published by Makris et al. 2011.
1Makris M, Watson HG: The Management of Coumarin-Induced Over-Anticoagulation Br. J. Haematol.2011; 114:271-280
Prothromplex Total Dose according to Initial INR Values | |
INR | Dose, [UI/kg] (UI refers to Factor IX) |
2.0-3.9 | 25 |
4.0-6.0 | 35 |
>6.0 | 50 |
The correction of vitamin K antagonist-induced deterioration of hemostasis persists for about 6-8 hours. However, the effects of vitamin K, if administered at the same time, are usually achieved within 4-6 hours. Therefore, it is not necessary to repeat treatment with human prothrombin complex when vitamin K has been administered.
As these recommendations are empirical and recovery and duration of effect may vary, INR monitoring during treatment is mandatory.
Treatment of Hemorrhages and Perioperative Prophylaxis of Bleeding in Congenital Deficiency of One of the Vitamin K-Dependent Coagulation Factors, when a Specific Coagulation Factor Product is not Available:
The calculation of the required dose for treatment is based on the empirical data that approximately 1 UI of factor VII or factor IX per kg of body weight increases the plasma activity of factor IX by about 0.015 UI/ml; and 1 UI per kg of body weight of factor VII by about 0.024 UI/ml. One UI of factor II or X per kg of body weight increases the plasma activity of factor II or X by about 0.021 UI/ml.
2Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers.Thromb Haemost. 2007;98(4):790-797
The dose of a specific factor administered is expressed in International Units (UI), which are related to the current WHO standard for each factor. The plasma activity of a specific coagulation factor is expressed either as a percentage (relative to normal plasma) or in International Units (relative to the international standard for the specific coagulation factor).
One International Unit (UI) of a coagulation factor activity is equivalent to the amount contained in 1 ml of normal human plasma. For example, the calculation of the required dose of factor X is based on the empirical data that 1 International Unit (UI) of factor X per kg of body weight increases the plasma activity of factor X by about 0.017 UI/ml. The required dose is determined using the following formula:
Required Units = body weight (kg) x desired increase in factor X (UI/ml) x 60
where 60 (ml/kg) is the reciprocal of the estimated recovery.
If the individual recovery is known, this value should be used in the calculation.
Maximum Single Dose
To correct the INR value, it is not necessary to exceed a dose of 50 IU/kg. If the severity of the hemorrhage requires a higher dose, the physician must evaluate the risk/benefit.
Pediatric population
The safety and efficacy of using Prothromplex Total in the pediatric population have not been established in clinical trials.
Interaction with other medications and other forms of interaction
If high doses of Prothromplex Total are administered, the heparin contained in the product should be taken into account when performing coagulation analyses sensitive to heparin.
Incompatibilities
This medication should not be mixed with other medications, except with the solvent supplied.
As with all coagulation factor medications, the efficacy and tolerance of the medication may be affected if mixed with other medications. It is recommended to flush a common venous access with isotonic saline solution before and after administration of Prothromplex Total.
Special precautions for disposal and other handling
Only the supplied reconstitution equipment should be used for reconstitution.
Prothromplex Total should be reconstituted immediately before administration. The solution should be used immediately thereafter (the solution does not contain preservatives).
The solution is transparent or slightly opalescent. The reconstituted product should be visually inspected before administration to verify the absence of foreign particles or discoloration. Do not use turbid solutions or those containing deposits.
N
It is strongly recommended that each time Prothromplex Total is administered to a patient, the name and batch number of the product be recorded to maintain a link between the patient and the product batch.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROTHROMPLEX TOTAL 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1000 IU/vialActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 500 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Prothya Biosolutions Netherlands B.V.Prescription required
Online doctors for PROTHROMPLEX TOTAL 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about PROTHROMPLEX TOTAL 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions