
BERIPLEX 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use BERIPLEX 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Beriplex 500 UI
Powder and solvent for solution for injection
Human prothrombin complex
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the pack
- What is Beriplex and what is it used for
- What you need to know before you use Beriplex
- How to use Beriplex
- Possible side effects
- Storage of Beriplex
- Contents of the pack and other information
1. What is BERIPLEX and what is it used for
What is Beriplex
Beriplex is presented as a powder and solvent. It is a white or slightly colored powder or friable solid. The prepared solution should be administered by injection into a vein.
Beriplex is prepared from human plasma (the liquid part of the blood) and contains human coagulation factors II, VII, IX, and X. Concentrates containing these coagulation factors are called prothrombin complex products. Coagulation factors II, VII, IX, and X are vitamin K-dependent and are important for blood coagulation. The lack of any of these factors means that the blood does not clot as quickly as it should, and there is an increased tendency to bleed. Replacement of factors II, VII, IX, and X with Beriplex repairs the coagulation mechanisms.
What is Beriplex used for
Beriplex is used for the prophylaxis (during surgery) and treatment of bleeding caused by acquired or congenital deficiency of vitamin K-dependent coagulation factors, i.e., factors II, VII, IX, and X in the blood, when specific coagulation factor products are not available.
2. What you need to know before you use Beriplex
The following sections contain information that your doctor should consider before administering Beriplex to you.
Do not use Beriplex
- If you are allergic to the active substances or to any of the other components of this medicine (listed in section 6).
Tell your doctor if you are allergic to any medicine or food.
- If you are more prone to blood clots than usual (patients at risk of disseminated intravascular coagulation).
- If you show an allergic response to heparin that causes a drop in blood platelet count (heparin-induced thrombocytopenia type II, HIT type II).
Tell your doctor or pharmacist if you have any of these diseases.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Beriplex if:
- Acquired deficiency of vitamin K-dependent coagulation factors: this may be induced by medications that inhibit the effect of vitamin K. Beriplex should only be used when rapid correction of prothrombin complex levels is necessary, e.g., in case of severe bleeding or emergency surgery.
- Congenital deficiency of any of the vitamin K-dependent factors: in this case, you should use specific coagulation factor products if available.
- Allergic or anaphylactic reactions (a severe allergic reaction that causes significant breathing difficulties or dizziness): administration of Beriplex should be stopped immediately (e.g., by stopping the injection).
- Increased risk of blood clot formation in a blood vessel (thrombosis), especially:
- If you have had a heart attack (history of coronary heart disease or myocardial infarction).
- If you have liver disease.
- If you have been operated on recently (pre- or post-operative patients).
- In newborns (neonates).
- If you are more prone to blood clot formation than usual (patients at risk of thromboembolic events or disseminated intravascular coagulation or simultaneous deficiency of coagulation inhibitors).
- Increased risk of coagulation due to increased consumption of platelets or blood coagulation factors. Treatment with Beriplex should only be started once the underlying cause has been treated.
- Decreased platelet production due to heparin (heparin-induced thrombocytopenia, HIT type II). Heparin, a protein with a blood clot-dissolving effect, is a component of Beriplex. Severe platelet count decrease can be associated with
- blood clots in a vein or leg,
- increased blood clot formation,
- skin rash at the injection site,
- petechiae (bleeding spots), and
- black stools (melena)
In these cases, the effect of heparin may be reduced (heparin tolerance). If such symptoms occur, you should stop using the product immediately and inform your doctor. In the future, you should not use products containing heparin.
- A special form of kidney inflammation has been reported after treatment in patients with hemophilia B and factor IX inhibitors. It was known that these patients had a history of allergic reactions.
Your doctor will carefully weigh the benefits of treatment with Beriplex against the risk of these complications.
Viral safety
When medicines are prepared from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These measures include:
- careful selection of blood and plasma donors to ensure that those who may be at risk of carrying infections are excluded,
- testing of each donation and plasma pools for signs of viruses or infections,
- inclusion of steps in the processing of blood or plasma that can inactivate or eliminate viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of transmitting an infection cannot be completely excluded. This also applies to unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for the non-enveloped hepatitis A virus and parvovirus B19.
It is possible that your doctor may recommend vaccination against hepatitis A and B if you are treated regularly/repeatedly with prothrombin complex products derived from human plasma.
It is strongly recommended that each time you are administered a dose of Beriplex, the name and batch number of the medicine should be recorded, in order to maintain a record of the batches used.
Using Beriplex with other medicines
- Tell your doctor or pharmacist that you are using or have recently used or might use any other medicines.
- Beriplex may inhibit the effect of vitamin K antagonist treatment. No interactions with other medicines are known.
- This medicine should not be mixed with other medicines, except those mentioned in section 6.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
- During pregnancy and breastfeeding, Beriplex should only be used if clearly indicated.
- No data are available on fertility.
Driving and using machines
No studies have been performed on the ability to drive or use machinery.
Beriplex contains sodium
Patients on low-sodium diets should be aware that this medicine contains up to 343 mg of sodium (approximately 15 mmol) per 100 ml. This should be taken into consideration by patients on a controlled sodium diet.
3. How to use Beriplex
Treatment should be initiated and supervised by a doctor experienced in the treatment of this type of disorder.
Posology
The amount of Factor II, VII, IX, and X you need and the duration of treatment will depend on various factors, such as your body weight, the severity and nature of your disease, the location and severity of the bleeding, or the need to prevent bleeding during surgery or investigation (see the section "This information is intended only for healthcare professionals").
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Overdose
Your doctor will regularly check your blood coagulation status during treatment. High doses of prothrombin complex concentrate have been associated with heart attacks, disseminated intravascular coagulation, and increased blood clot formation in blood vessels in patients at risk of these complications.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been commonlyobserved (may affect up to 1 in 10 patients):
- Risk of blood clot formation (see section 2).
- Headache.
- Increased body temperature.
The following side effects have been uncommonlyobserved (may affect up to 1 in 100 patients):
- Hypersensitivity or allergic reactions (see section 2).
The frequency of the following side effects is not known(cannot be estimated from the available data):
- Excessive coagulation resulting in severe bleeding.
- Anaphylactic reactions including shock (see section 2).
- Development of circulating antibodies that inhibit one or more coagulation factors.
Pediatric population
No data are available on the use of Beriplex in the pediatric population.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BERIPLEX
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and on the carton after EXP.
- Do not store above 25 °C.
- Do not freeze.
- Keep the container in the outer packaging to protect it from light.
- Beriplex does not contain preservatives, so the prepared solution should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Beriplex Composition
Beriplex 500 IU contains 400 – 620 IU of human coagulation factor IX per vial.
The active principle is:
A concentrate of human coagulation factors II, VII, IX, and X, and proteins C and S.
Other components are:
Human antithrombin III, heparin, human albumin, sodium chloride, sodium citrate, HCl or NaOH (in small quantities to adjust pH).
Solvent:Water for injectable preparations.
Product Appearance and Container Content
Beriplex is presented as a white or slightly colored powder and is supplied with water for injectable preparations as a solvent. The powder must be dissolved in 20 ml of water for injectable preparations.
The prepared solution must be transparent or slightly opalescent, i.e., it may present bubbles when placed in front of light but must not contain detectable particles.
Presentation
A container with 500 IU that contains:
- 1 vial with powder
- 1 vial with 20 ml water for injectable preparations
- 1 transfer device with 20/20 filter
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Barcelona, Spain
This medication is authorized in the member states of the European Economic Area under the following names:
Austria | Beriplex P/N 500 I.E. Powder and solvent for solution for injection |
Belgium | Confidex 500 I.E., powder and solvent for solution for injection |
Bulgaria | Beriplex P/N 500, 500 IU, Powder and solvent for solution for injection |
Croatia | Beriplex P/N 500 IU powder and solvent for solution for injection |
Czech Republic Denmark | Beriplex 500 IU Confidex |
Finland | Confidex 500 IU injection dry substance and solvent, solution |
France | Confidex 500 UI, powder and solvent for injectable solution |
Germany | Beriplex P/N 500 |
Greece | Beriplex P/N Κ?νις και διαλ?της για εν?σιμο δι?λυμα 500 IU/vial |
Hungary Ireland | Beriplex P/N 500 powder and solvent for injectable solution Beriplex P/N 500 IU, powder and solvent for solution for injection |
Italy | Confidex 500 |
Luxembourg | Confidex 500 UI powder and solvent for injectable solution |
Malta Netherlands | Beriplex P/N 500, powder and solvent for solution for injection Beriplex P/N 500 IE, powder and solvent for solution for injection |
Norway | Confidex 500 IU powder and liquid for injectable solution |
Poland Portugal | Beriplex P/N 500 Beriplex 500 UI powder and solvent for injectable solution |
Romania Slovakia Slovenia Spain | Beriplex P/N 500 UI powder and solvent for injectable solution Beriplex 500 IU Beriplex P/N 500 i.e. powder and vehicle for solution for injection Beriplex 500 UI powder and solvent for injectable solution |
Sweden | Confidex 500 IE, powder and liquid for injectable solution |
United Kingdom | Beriplex P/N 500 IU, powder and solvent for solution for injection |
Date of the last revision of this prospectus: July 2017
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)
http://www.aemps.gob.es
This information is intended only for healthcare professionals:
Qualitative and Quantitative Composition
The nominal content of human coagulation factors is expressed in the following table, expressed in IU:
Component Name | Content after reconstitution (IU/ml) | Beriplex 500 IUcontent per vial (IU) |
Active Principles | ||
Human coagulation factor II | 20 – 48 | 400 – 960 |
Human coagulation factor VII | 10 – 25 | 200 – 500 |
Human coagulation factor IX | 20 – 31 | 400 – 620 |
Human coagulation factor X | 22 – 60 | 440 – 1200 |
Other Active Principles | ||
Protein C | 15 – 45 | 300 – 900 |
Protein S | 12 - 38 | 240 - 760 |
The total protein content is 6 – 14 mg/ml of reconstituted solution.
The specific activity expressed in factor IX is 2.5 IU/mg of total protein.
The activity of all coagulation factors, as well as proteins C and S (antigen), has been tested according to current international standards established by the World Health Organization (WHO).
Posology and Method of Administration
Posology
The following dosage guidelines are of a general nature only.
The dosage and frequency of administration will be established individually for each patient. The dosing intervals must be adapted to the circulating half-lives of the respective coagulation factors of the prothrombin complex. Individual dosage requirements can only be identified based on periodic determination of plasma levels of the relevant coagulation factors or analysis of the overall levels of the prothrombin complex (INR, Quick test) and continuous monitoring of the patient's clinical situation.
In the case of major surgical interventions, precise monitoring of substitution therapy is essential, by means of coagulation analysis (specific factor assays and/or global tests to measure prothrombin complex levels).
- Bleeding and perioperative prophylaxis of bleeding during treatment with vitamin K antagonists:
The dosage will depend on the INR value before treatment and the INR value to be achieved. The INR value before treatment should be taken as close as possible to the time of administration to calculate the adequate dose of Beriplex. The following table provides approximate doses (ml of reconstituted product solution/kg of body weight and IU of factor IX/kg of body weight) required to normalize the INR (e.g., < 1.3) at different initial INR levels.
INR before treatment | 2.0 – 3.9 | 4.0 – 6.0 | > 6.0 |
Approximate dose (ml/kg of body weight) | 1 | 1.4 | 2 |
Approximate dose of factor IX (IU)/kg of body weight | 25 | 35 | 50 |
The dose is based on body weight up to, but not exceeding, 100 kg. For patients whose body mass exceeds 100 kg, the maximum single dose (IU of factor IX) must not exceed 2,500 IU for an INR of 2.0 – 3.9, 3,500 IU for an INR of 4.0 – 6.0, and 5,000 IU for an INR > 6.0.
Correction of the hemostasis disorder induced by vitamin K antagonists is commonly achieved approximately 30 minutes after injection. Simultaneous administration of vitamin K should be considered in patients treated with Beriplex for the urgent neutralization of vitamin K antagonists, as the effect of vitamin K is normally achieved after 4 to 6 hours. Repeated administration of Beriplex is not recommended in patients who require urgent treatment for neutralization of vitamin K antagonists, as it is not supported by clinical data.
These recommendations are based on data from clinical trials with a limited number of individuals. Recovery and duration of effect may vary, so monitoring of INR during treatment is mandatory.
- Bleeding and perioperative prophylaxis in congenital deficiency of some vitamin K-dependent coagulation factors, when specific factor products are not available.
The calculation of the required dose of prothrombin complex concentrate is based on clinical trial data:
?1 IU of factor IX per kilogram of body weight may increase plasma activity of factor IX by 1.3% (0.013 IU/ml) of normal activity.
?1 IU of factor VII per kg of body weight increases plasma activity of factor VII by 1.7% (0.017 IU/ml) of normal activity.
?1 IU of factor II per kg of body weight increases plasma activity of factor II by 1.9% (0.019 IU/ml) of normal activity.
?1 IU of factor X per kg of body weight increases plasma activity of factor X by 1.9% (0.019 IU/ml) of normal activity.
The dosage of a specific factor administered is expressed in International Units (IU), which are related to the current WHO standard for each factor. The plasma activity of a specific coagulation factor is expressed as a percentage (relative to normal plasma) or in International Units (relative to the international standard for the specific coagulation factor).
One International Unit (IU) of coagulation factor activity is equivalent to the amount contained in 1 ml of normal human plasma.
For example, the calculation of the required dose of factor X is based on the finding that 1 International Unit (IU) of factor X per kg of body weight increases plasma activity of factor X by 0.019 IU/ml.
The required dose is determined using the following formula:
Required Units = body weight [kg] x desired increase in factor X [IU/ml] x 53, where 53 (ml/kg) is the reciprocal of the estimated recovery.
Note that the calculation is based on data from patients who received vitamin K antagonists. A calculation based on data from healthy volunteers would provide a lower estimate of the required dose.
If the individual recovery is known, this value should be used in the calculation.
Product-specific information is available from clinical trials with healthy volunteers (N = 15), from the neutralization of vitamin K antagonists in the treatment of severe bleeding or perioperative prophylaxis of bleeding (N = 98, N = 43).
Pediatric Population
The safety and efficacy of Beriplex in children and adolescents have not been established through controlled clinical trials.
Geriatric Population
The dosage and method of administration in elderly patients (over 65 years) correspond to the general recommendations.
Method of Administration
General Instructions
- The solution must be transparent or slightly opalescent. After filtration/extraction (see below) and before administration, the reconstituted product must be visually inspected for the presence of foreign particles and discoloration.
Do not use turbid solutions or those containing residues.
- Reconstitution and extraction must be performed under aseptic conditions.
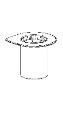
Reconstitution
Bring the solvent to room temperature. Check that the seals have been removed from the product and solvent vials and that the stoppers have been treated with an antisptic solution, allowing it to dry before opening the Mix2Vial device package.
|
|
|
|
|
|
|
|
|
Discard the solvent vial with the blue Mix2Vial adapter attached. |
|
|
|
|
Extraction and Administration
8 |
|
9 |
|
Precautions should be taken to prevent blood from entering the syringe loaded with the product, as there is a risk that the blood may coagulate in the syringe and that fibrin clots may be administered to the patient.
If more than one vial of Beriplex is needed, it is possible to pool several vials of Beriplex in a single infusion using a commercially available infusion set.
The Beriplex solution must not be diluted.
The reconstituted solution must be administered intravenously (at a maximum rate of 8 ml/minute*).
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
Special Warnings and Precautions for Use
No data are available on the use of Beriplex in the case of perinatal bleeding due to vitamin K deficiency in neonates.
Guidelines for platelet count monitoring:
Carefully monitor the platelet count.
Interaction with Other Medicinal Products and Other Forms of Interaction
When performing coagulation tests sensitive to heparin in patients treated with high doses of human prothrombin complex, the heparin contained in the administered product should be considered.
*In clinical trials with Beriplex, doses were administered with a maximum infusion rate of 0.12 ml/kg/min to patients with a weight <70 kg.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BERIPLEX 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1000 IU/vialActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Prothya Biosolutions Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 500 IUActive substance: coagulation factor IX, II, VII and X in combinationManufacturer: Prothya Biosolutions Netherlands B.V.Prescription required
Online doctors for BERIPLEX 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about BERIPLEX 500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





 1
1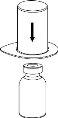 2
2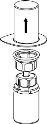 3
3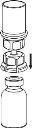 4
4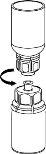 5
5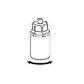 6
6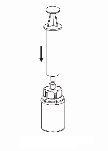 7
7









