
Beriplex P/n 250
Ask a doctor about a prescription for Beriplex P/n 250

How to use Beriplex P/n 250
Patient Information Leaflet
Beriplex P/N 250
Powder and solvent for solution for injection
Human prothrombin complex
Read the leaflet carefully before using the medicine, as it contains important information for you.
- Keep this leaflet, you may need to read it again
- If you have any further questions, ask your doctor or pharmacist
- This medicine has been prescribed for you, do not pass it on to others
- The medicine may harm others, even if their symptoms are the same as yours
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Contents of the pack:
- 1. What Beriplex P/N 250 is and what it is used for
- 2. Important information before using Beriplex P/N 250
- 3. How to use Beriplex P/N 250
- 4. Possible side effects
- 5. How to store Beriplex P/N 250
- 6. Contents of the pack and other information
1. What Beriplex P/N 250 is and what it is used for
What Beriplex P/N 250 is
Beriplex P/N 250 is available as a powder with a solvent provided. It is a white or slightly colored powder or a brittle solid mass. The reconstituted solution is for intravenous injection.
Beriplex P/N 250 is made from human plasma (the liquid part of blood) and contains human clotting factors II, VII, IX, and X. Concentrates containing these clotting factors are called prothrombin complex products. Vitamin K-dependent clotting factors II, VII, IX, and X play an important role in the blood clotting process (coagulation). A deficiency of any of these clotting factors can cause the blood to clot more slowly than it should, and the risk of bleeding is greater. Replacing clotting factors II, VII, IX, and X with Beriplex P/N 250 leads to the correction of the clotting mechanism.
What Beriplex P/N 250 is used for
Beriplex P/N 250 is used to prevent (during surgical procedures) and treat bleeding caused by acquired or congenital deficiency of vitamin K-dependent clotting factors II, VII, IX, and X, when a medicine containing a specific clotting factor is not available.
2. Important information before using Beriplex P/N 250
The following section contains information that you and your doctor should read before using Beriplex P/N 250.
When not to use Beriplex P/N 250:
if you are allergic to the active substances or any of the other ingredients of this medicine (listed in section 6).
Tell your doctor if you are allergic to any medicines or foods.
- if there is an increased risk of forming blood clots (patients with a risk of disseminated intravascular coagulation)
- in case of an allergic reaction to heparin, causing a decrease in platelet count (heparin-induced thrombocytopenia type II, HIT type II).
Tell your doctor or pharmacist if you have any of these conditions.
Warnings and precautions
Before starting treatment with Beriplex, discuss it with your doctor or pharmacist.
- In case of acquired deficiency of vitamin K-dependent clotting factors. This may be caused by taking medicines that block the action of vitamin K. Beriplex P/N 250 may only be used if it is necessary to quickly correct the level of clotting factors, e.g., in case of severe bleeding or emergency surgery.
- In case of congenital deficiency of any of the vitamin K-dependent clotting factors, a medicine containing a specific clotting factor should be used whenever possible.
- In case of an allergic or anaphylactic reaction (a severe allergic reaction causing serious breathing difficulties or dizziness):
Beriplex P/N 250 administration should be stopped immediately (e.g., stop the injection).
- If there is a risk of increased blood clot formation in blood vessels (thrombosis), especially in the following cases:
- in patients after a heart attack (with diagnosed coronary artery disease or myocardial infarction)
- in patients with liver disease
- in patients immediately before and after surgical procedures
- in newborns
- in patients with an increased risk of blood clot formation (patients with a risk of thromboembolic disease or disseminated intravascular coagulation, or with a deficiency of clotting inhibitors)
- If there is an increased risk of bleeding disorders caused by increased consumption of platelets or clotting factors. Treatment with Beriplex P/N 250 may be started after the underlying disease has been treated.
- Heparin-induced reduction in platelet count (heparin-induced thrombocytopenia, HIT type II). Heparin, a protein that dissolves blood clots, is a component of Beriplex. A significant decrease in platelet count may be associated with:
- formation of blood clots in the veins of the lower limbs,
- increased tendency to form blood clots,
- in some cases, a skin rash at the injection site,
- bruising and
- the appearance of black stools. In such cases, the action of heparin may be impaired (heparin tolerance). If these symptoms occur, the administration of the medicine should be stopped immediately and a doctor should be consulted. In the future, medicines containing heparin should not be taken.
- A special form of kidney inflammation has been reported after treatment of patients with hemophilia B and inhibitors of factor IX. These patients had a history of allergic reactions.
Your doctor should consider the benefits of Beriplex P/N 250 therapy against the risk of these complications.
Virus safety
When medicines are obtained from human blood or plasma, various methods are used to prevent the transmission of infectious agents to the patient. These include:
- proper selection of blood and plasma donors to exclude the possibility of transmitting infectious agents,
- testing of individual donors and plasma pools for viruses and other infections,
- inclusion of steps in the manufacturing process to inactivate or remove viruses.
Despite these precautions, when using medicines obtained from human blood or plasma, it is not possible to completely exclude the risk of infectious diseases due to the possibility of transmitting infectious agents. This also applies to unknown pathogens and other types of infections.
The methods used are considered effective against such enveloped viruses as human immunodeficiency virus (HIV, the virus that causes AIDS), hepatitis B virus, hepatitis C virus (viral hepatitis), and non-enveloped hepatitis A virus (viral hepatitis) and parvovirus B19.
Your doctor may recommend appropriate vaccination against hepatitis A and B if you regularly/repeatedly take medicines obtained from human plasma.
It is strongly recommended that each administration of Beriplex P/N 250 be recorded in the patient's medical record, including the name and batch number of the medicine, to document the batches used.
Beriplex P/N 250 and other medicines
- Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
- Beriplex P/N 250 may inhibit the action of vitamin K antagonists. Interactions with other medicines are not known.
- Beriplex P/N 250 should not be mixed with other medicines, except for those listed in section 6.
Pregnancy, breastfeeding, and fertility
- If you are pregnant, breastfeeding, or think you may be pregnant, or if you plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- In pregnant or breastfeeding women, Beriplex P/N 250 should only be used if necessary.
- There are no available data on the effect on fertility.
Driving and using machines
There are no adequate studies to determine the effect of the medicine on driving and using machines.
Beriplex P/N 250 contains sodium
Beriplex P/N 250 contains up to 343 mg of sodium (approximately 15 mmol) per 100 ml. This should be taken into account for patients on a controlled sodium diet.
3. How to use Beriplex P/N 250
Treatment should be started under the supervision of a doctor experienced in the treatment of this type of disorder.
Dosage
The recommended dose of clotting factors II, VII, IX, and X, as well as the duration of treatment, depends on several factors, such as body weight, disease severity and progression, bleeding location and intensity, and the need to prevent bleeding during surgery or patient diagnosis (see "Information intended for healthcare professionals only").
In case of any doubts about the use of the medicine, consult your doctor or pharmacist.
Overdose
During treatment, your doctor should regularly monitor your blood clotting parameters.
Administration of high doses of prothrombin complex concentrate has been associated with myocardial infarction, disseminated intravascular coagulation, and blood clot formation in blood vessels in patients at increased risk of these disorders.
4. Possible side effects
Like all medicines, Beriplex P/N 250 can cause side effects, although not everybody gets them.
The following side effects have been commonlyobserved (in less than 1 in 10 patients):
- Risk of increased blood clot formation (see section 2)
- Headache
- Increased body temperature
The following side effects have been less commonlyobserved (in less than 1 in 100 patients):
- Hypersensitivity or allergic reactions (see section 2)
The frequency of the following side effects is unknown(cannot be determined from available data):
- Severe bleeding due to excessive clotting
- Anaphylactic reactions, including shock (see section 2)
- Formation of circulating antibodies that inhibit one or more clotting factors
Children and adolescents
There are no data on the use of Beriplex in children and adolescents.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Beriplex P/N 250
- Keep the medicine out of the sight and reach of children.
- Do not use Beriplex P/N 250 after the expiry date stated on the label and carton.
- Do not store above 25°C.
- Do not freeze.
- Store the vial in the outer carton to protect from light.
- Beriplex P/N 250 does not contain preservatives, the reconstituted product should be used immediately.
6. Contents of the pack and other information
What Beriplex P/N 250 contains
Beriplex P/N 250 contains 200 - 310 IU of human factor IX in a vial.
The active substance is:
Concentrate of human clotting factors II, VII, IX, and X, proteins C and S.
Other ingredients are:
Human antithrombin III, heparin, human albumin, sodium chloride, sodium citrate, HCl or NaOH (in small amounts to adjust pH).
Solvent: Water for injections.
What Beriplex P/N 250 looks like and contents of the pack
Beriplex P/N 250 is a white or slightly colored powder and is provided with water for injections as a solvent. The powder should be dissolved in 10 ml of water for injections.
The reconstituted solution should be clear or slightly opalescent, which means it may shine when viewed in light, and should not contain any solid particles.
Pack sizes
A pack with a dose of 250 IU contains:
- 1 vial of powder
- 1 vial of water for injections (10 ml)
- 1 Mix2Vial 20/20 transfer system with filter
Name and address of the marketing authorization holder and manufacturer:
CSL Behring GmbH
Emil-von-Behring-Str. 76
35041 Marburg
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names
Austria
Beriplex P/N 250 I.E. Pulver und Lösungsmittel zur Herstellung einer Injektionslösung
Denmark
Confidex
Finland
Confidex 250 IU injektiokuiva-aine ja liuotin, liuosta varten
France
Confidex 250 UI, poudre et solvant pour solution injectable
Germany
Ireland
Beriplex P/N 250
Beriplex P/N 250, powder and solvent for solution for injection
Netherlands
Beriplex P/N 250 IE, poeder en oplosmiddel voor oplossing voor injectie
Norway
Poland
Confidex 250 IU pulver og væske til injeksjonsvæske, oppløsning
Beriplex P/N 250
Sweden
Confidex 250 IE, pulver och vätska till injektionsvätska, lösning
United Kingdom
Beriplex P/N 250 IU, powder and solvent for solution for injection
Date of last revision of the leaflet:
Information intended for healthcare professionals only
Quantitative and qualitative composition
Beriplex P/N 250 nominally contains the following amounts of IU of human clotting factors, as shown in the table below:
| Component name | Content after reconstitution (IU/ml) | Content in one vial of Beriplex P/N 250 (IU) |
| Active substances | ||
| Human factor II |
|
|
| Human factor VII |
|
|
| Human factor IX |
|
|
| Human factor X |
|
|
| Other active substances | ||
| Protein C |
| 150 -450 |
| Protein S |
|
|
The total protein content after reconstitution is 6-14 mg/ml in the solution.
The specific activity of factor IX is 2.5 IU per mg of total protein.
The activity of all clotting factors, as well as proteins C and S (antigens), was tested according to current international WHO standards.
Dosage and administration Dosage
The following are only general dosage guidelines.
The dose and frequency of administration should be calculated for each patient individually. The intervals between doses should be adjusted according to the half-life of the individual clotting factors of the prothrombin complex. The individual dose is determined based on regular measurement of the activity of individual clotting factors in the blood or based on laboratory tests that generally determine the activity of the prothrombin complex (INR, Quick test) and through continuous monitoring of the patient's clinical condition.
In the case of major surgical interventions, careful monitoring of replacement therapy (tests for individual clotting factors and general tests for clotting factors of the prothrombin complex) is necessary.
- Bleeding and perioperative bleeding prophylaxis during treatment with vitamin K antagonists::
The dose of the medicine depends on the INR value measured before treatment and the INR value that the patient should achieve. The INR value before treatment should be measured as soon as possible before administration of the medicine to allow calculation of the appropriate dose of Beriplex. The following table shows the approximate doses (ml/kg body weight of reconstituted product and IU of factor IX/kg body weight) to achieve normalization of the INR (i.e., INR ≤ 1.3) at different initial INR values.
| INR value before treatment | 2.0 – 3.9 | 4.0 – 6.0 | >6.0 |
| Approximate dose in ml/kg body weight | 1 | 1.4 | 2 |
| Approximate dose in IU (factor IX) per kilogram of body weight | 25 | 35 | 50 |
Dosing is based on body weight, provided it does not exceed 100 kg. In patients with a body weight over 100 kg, the maximum single dose (IU of factor IX) should not exceed 2500 IU for an INR of 2.0-3.9; 3500 IU for an INR of 4.0-6.0; and 5000 IU for an INR > 6.0.
Normalization of hemostasis disorders caused by vitamin K antagonists is usually achieved within 30 minutes after injection. Concurrent administration of vitamin K should be considered in patients receiving Beriplex for urgent reversal of vitamin K antagonists, as the effect of vitamin K is usually achieved within 4-6 hours. Repeated dosing of Beriplex in patients requiring urgent reversal of vitamin K antagonists is not supported by clinical studies and is therefore not recommended.
These recommendations are based on clinical studies in a limited number of individuals.
It is necessary to monitor the INR value during treatment, as the efficacy and duration of action may vary between patients.
- Bleeding and perioperative bleeding prophylaxis in patients with congenital deficiencies of any of the vitamin K-dependent clotting factors, when the use of a specific clotting factor preparation is not possible
Calculation of the required dose of prothrombin complex concentrate based on clinical studies:
- 1 IU of factor IX per kg body weight is expected to increase the activity of factor IX in the blood by 1.3% (0.013 IU/ml) compared to normal,
- 1 IU of factor VII per kg body weight is expected to increase the activity of factor VII in the blood by 1.7% compared to normal (0.017 IU/ml),
- 1 IU of factor II per kg body weight is expected to increase the activity of factor II in the blood by 1.9% compared to normal (0.019 IU/ml),
- 1 IU of factor X per kg body weight is expected to increase the activity of factor X in the blood by 1.9% compared to normal (0.019 IU/ml).
The dose of each clotting factor is expressed in international units (IU), in accordance with the applicable WHO standard for each clotting factor. The activity of the clotting factor in the blood is expressed as a percentage (compared to normal human blood) or in international units (according to the international standard for the specific clotting factor in the blood).
One international unit (IU) of clotting factor activity is equal to the activity of that factor contained in 1 ml of normal human blood.
For example, the calculation of the required dose of factor X is based on the empirical finding that 1 IU of factor X per kg body weight increases the activity of factor X in the blood by 0.019 IU/ml.
The required dose is calculated using the following formula:
Required dose (IU) = body weight (kg) x desired increase in factor X activity (IU/ml) x 53,
where 53 (ml/kg) is the inverse of the estimated recovery value.
Note that these calculations are based on data from patients treated with vitamin K antagonists. Calculations based on data from healthy individuals would provide a lower required dose.
If the individual recovery value is known, it should be used for calculations.
Data on the product are available based on clinical studies conducted in healthy volunteers (N=15), in the reversal of vitamin K antagonists in the treatment of acute major bleeding or perioperative bleeding prophylaxis. (N=98, N=43)
Children and adolescents
The safety and efficacy of Beriplex P/N 250 in children and adolescents have not been established in controlled clinical trials.
Elderly
Dosage and administration in the elderly (> 65 years) are in line with the general recommendations.
Method of administration General instructions
- The solution should be clear or slightly opalescent. After filtration and withdrawal of the reconstituted solution (see below), before administration, check that it does not contain any visible particles or discoloration. Do not use cloudy solutions or those containing sediment or particles.
- Reconstitution and withdrawal from the vial should be carried out under aseptic conditions.
Reconstitution
Bring the solvent to room temperature.
Make sure the caps of the vials with the powder and solvent are removed, wipe the rubber stoppers with an aseptic solution, and let them dry before opening the Mix2Vial package.
1 |
|
2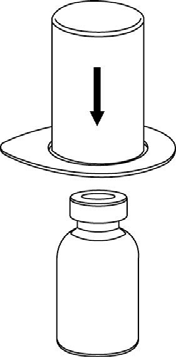 |
|
3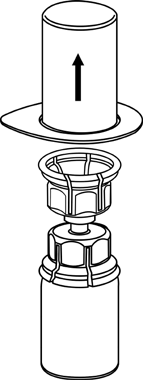 |
|
4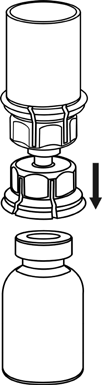 |
|
5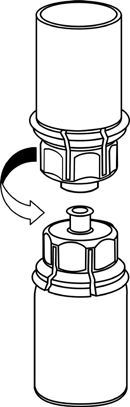 |
|
6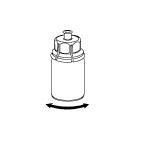 |
|
7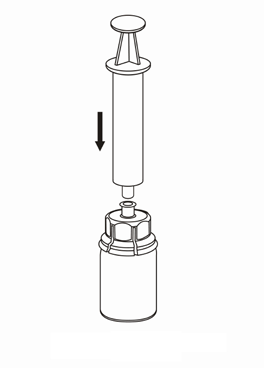 |
|
Withdrawal and administration
8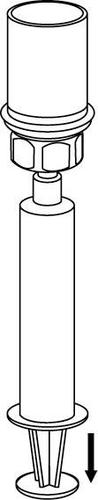 |
|
9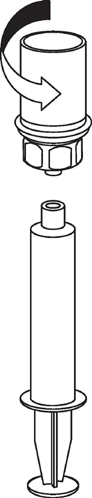 |
|
Be careful not to let blood enter the syringe filled with the product. This can cause a reaction of the clotting factors in the product and the formation of fibrin clots, which can be administered to the patient.
If more than one vial of Beriplex is required, there is a possibility of combining several vials for administration as a single infusion using a commercially available administration set.
The reconstituted Beriplex solution should not be diluted.
The reconstituted solution should be administered intravenously (no faster than 8 ml/min*).
Any unused product or waste material should be disposed of in accordance with local requirements.
Special warnings and precautions for use
There are no clinical data available on the use of Beriplex P/N 250 in postpartum hemorrhage due to vitamin K deficiency in newborns.
_________________________________________________
*In clinical studies of Beriplex, patients weighing less than 70 kg had recommended dosing with a maximum infusion rate of 0.12 ml/kg/min (less than 8 ml/min).
Notes on platelet count monitoring:
Platelet count should be closely monitored.
Interactions with other medicines and other types of interactions
In the case of performing coagulation tests sensitive to the presence of heparin, in patients receiving high doses of prothrombin complex concentrate, the heparin dose contained in the product should be taken into account in the test results.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterCSL Behring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Beriplex P/n 250Dosage form: Powder, 1000 IUActive substance: coagulation factor IX, II, VII and X in combinationPrescription requiredDosage form: Powder, 500 IUActive substance: coagulation factor IX, II, VII and X in combinationPrescription requiredDosage form: Powder, 500 IUActive substance: coagulation factor IX, II, VII and X in combinationPrescription required
Alternatives to Beriplex P/n 250 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Beriplex P/n 250 in Spain
Alternative to Beriplex P/n 250 in Ukraine
Online doctors for Beriplex P/n 250
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Beriplex P/n 250 – subject to medical assessment and local rules.














