PRIOSOL CON 2 mmol/L POTASSIUM HEMOFILTRATION SOLUTION


How to use PRIOSOL CON 2 mmol/L POTASSIUM HEMOFILTRATION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Priosol with 2 mmol/l Potassium is and what it is used for
- What you need to know before you are given Priosol with 2 mmol/l Potassium
- How to use Priosol with 2 mmol/l Potassium
- Possible side effects
- Storage of Priosol with 2 mmol/l Potassium
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
Priosol with 2 mmol/l Potassium Solution for Haemofiltration
Read all of this leaflet carefully before this medicine is administered to you, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What Priosol with 2 mmol/l Potassium is and what it is used for
- What you need to know before you are given Priosol with 2 mmol/l Potassium
- How to use Priosol with 2 mmol/l Potassium
- Possible side effects
- Storage of Priosol with 2 mmol/l Potassium
- Contents of the pack and further information
1. What Priosol with 2 mmol/l Potassium is and what it is used for
Priosol with 2 mmol/l Potassium is a solution for haemofiltration. It is used in patients with acute renal failure, when the kidneys are not able to remove waste products from the blood. Continuous haemofiltration is a procedure used to remove waste products from the body that would normally be excreted in the urine through the kidneys. The solution corrects the balance of fluids and ensures that after treatment, the loss of salts (electrolytes) is restored.
2. What you need to know before you are given Priosol with 2 mmol/l Potassium
Priosol with 2 mmol/l Potassium must not be given to you if:
- you have an abnormally low level of potassium in your blood (hypokalaemia)
- your blood contains abnormally low levels of acidic substances (metabolic alkalosis)
The haemofiltration procedure should not be used if you have
- renal failure together with a very high metabolic turnover (hyper-catabolic state), in this situation the accumulation of waste products in your body can no longer be corrected by haemofiltration
- poor blood flow at the site of insertion of the cannula in the vein
- a high risk of bleeding due to the fact that you are receiving medications to prevent blood clotting (systemic anticoagulation).
Warnings and precautions
Talk to your doctor or pharmacist before you are given Priosol with 2 mmol/l Potassium.
Before and during haemofiltration, your blood pressure and fluid balance, electrolyte balance, acid-base balance, and kidney function will be monitored. Your blood sugar and phosphate levels will be regularly checked.
In addition, your serum potassium levels will be monitored before and during haemofiltration.
Using Priosol with 2 mmol/l Potassium with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The level of other medicines in your blood may be reduced during haemofiltration, which your doctor will take into account.
Interactions with other medicines can be avoided with the use of the correct dose of the haemofiltration solution and careful monitoring.
The following interactions will be considered:
- Infusions given in intensive care may change the composition of the blood and its water status.
- The toxic effects of certain medicines used to treat heart failure (medicines containing digitalis) may not become apparent if your potassium or magnesium levels are too high, or your calcium levels are too low. When these levels are corrected by haemofiltration, these toxic effects may appear and cause, for example, heart rhythm abnormalities. If you have low potassium or high calcium levels in your blood, digitalis may produce toxic effects at doses lower than those normally used for treatment.
- Vitamin D and medicines containing calcium may increase the risk of elevated calcium levels in the blood (hypercalcaemia).
- The use of additional sodium hydrogen carbonate may increase the risk of low acid levels in the blood (metabolic alkalosis).
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before you are given this medicine.
There are no sufficient data on the use of haemofiltration solutions during pregnancy. However, since all the components of this medicine are natural substances that only replace the same substances that are lost from the body during haemofiltration, no risks to the child during pregnancy and breast-feeding, or effects on fertility, are expected.
Driving and using machines
This medicine is usually given to patients who are immobile in a hospital or dialysis unit, which excludes driving and using machines.
3. How to use Priosol with 2 mmol/l Potassium
This medicine will only be given to you under the direction of a doctor with experience in haemofiltration techniques.
Your doctor will decide the correct dose for you, taking into account your clinical condition, body weight, and metabolic state. Unless otherwise indicated, in patients of any age, a filtration rate of 20-25 ml/kg body weight per hour is recommended for the removal of substances that are normally excreted in the urine.
The haemofiltration solution will be given to you ready to use through the tube system of the haemofiltration equipment (extracorporeal circulation) using a perfusion pump.
Treatment of acute renal failure is carried out for a limited period of time and ends when kidney function is fully restored.
If you are given more Priosol with 2 mmol/l Potassium than recommended
No life-threatening situations have been described after administration of the prescribed dose of this medicine. Administration can be stopped immediately if necessary.
Unbalanced administration may lead to an excess or deficit of fluid in the body (hyperhydration or dehydration). This situation becomes apparent through changes in blood pressure or pulse.
If too much of the haemofiltration solution is administered, an overdose of sodium hydrogen carbonate may occur. This may result in abnormally low acid levels in the blood (metabolic alkalosis), a lower amount of dissolved calcium in the blood (decrease in ionized calcium), or muscle cramps (tetany).
An overdose can cause heart failure and/or pulmonary congestion and may disrupt the acid-base and electrolyte balance.
Your doctor will decide on the appropriate treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
No cases of side effects associated with this medicine have been reported to date. However, the following side effects may occur. The frequency of these side effects cannot be estimated from the available data:
Excess or deficit of fluid in the body (hyperhydration or dehydration), abnormal levels of salts (electrolytes), low phosphate levels in the blood (hypophosphataemia), high sugar levels in the blood (hyperglycaemia), abnormally low acid levels in the blood (metabolic alkalosis), high or low blood pressure (hypertension or hypotension), nausea, vomiting, and muscle cramps.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Priosol with 2 mmol/l Potassium
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bag and carton after “EXP”. The expiry date is the last day of the month stated.
Storage conditions
Do not store above 25°C.
Do not refrigerate or freeze.
Storage conditions after preparation of the ready-to-use solution
The mixed product should be used immediately. The mixed product is physically and chemically stable for 24 hours at 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Priosol with 2 mmol/l Potassium
The active substances are: | Small chamber Electrolyte solution | Large chamber Bicarbonate solution | ||
555 ml contain | per 1,000 ml | 4,445 ml contain | per 1,000 ml | |
Sodium chloride | 2.34 g | 4.21 g | 27.47 g | 6.18 g |
Potassium chloride | 0.74 g | 1.34 g | — | — |
Calcium chloride dihydrate | 1.10 g | 1.98 g | — | — |
Magnesium chloride hexahydrate | 0.51 g | 0.91 g | — | — |
Glucose monohydrate equivalent to anhydrous glucose | 5.49 g 5.0 g | 9.90 g 9.0 g | — | — |
Sodium hydrogen carbonate | — | — | 15.96 g | 3.59 g |
Electrolytes: | [mmol/chamber] | [mmol/l] | [mmol/chamber] | [mmol/l] |
Na+ | 40.0 | 72 | 660 | 149 |
K+ | 10.0 | 18.0 | — | — |
Ca2+ | 7.5 | 13.5 | — | — |
Mg2+ | 2.5 | 4.5 | — | — |
Cl- | 85.0 | 153 | 470 | 106 |
HCO3- | — | — | 190 | 42.8 |
Theoretical osmolality [mOsm/l] | 311 | 297 |
Composition of the ready-to-use haemofiltration solution after mixing:
1,000 ml of the ready-to-use haemofiltration solution contain [mmol/l]:
Na+ 140
K+2.0
Ca2+ 1.5
Mg2+ 0.5
Cl- 111
HCO3- 35.0
Anhydrous glucose 5.6 (equiv. to 1.0 g)
Theoretical osmolality [mOsm/l] 296
pH 7.0-8.0
The other ingredients are:
Electrolyte solution (small chamber)
Hydrochloric acid 25% (for pH adjustment), water for injections
Bicarbonate solution (large chamber)
Carbon dioxide (for pH adjustment), water for injections
Appearance and pack contents
Haemofiltration solution.
Clear and colourless solution, free from visible particles.
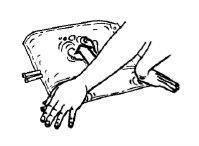
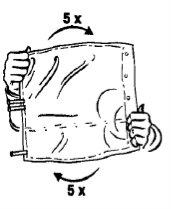
This medicine is provided in a two-chamber bag. When the two solutions are mixed by opening the peel seal between the two chambers, the ready-to-use haemofiltration solution is obtained.
Two bags of 5,000 ml (two-chamber bags, 4,445 ml and 555 ml) per carton.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
- Braun Avitum AG
Schwarzenberger Weg 73-79
34212 Melsungen Germany
Manufacturer
- Braun Avitum AG
Kattenvenner Str. 32
49219 Glandorf
Germany
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria, Germany, Luxembourg: Duosol mit 2 mmol/l Kalium Hämofiltrationslösung
Bulgaria: ?????? 2 mmol/l ?????, ??????? ?? ?????????????
Croatia: Duosol s 2 mmol/l kalija otopina za hemofiltraciju
Czech Republic: Duosol s 2 mmol/l kalia
Denmark, Norway, Sweden: Duosol Kalium 2 mmol/l
Estonia: Duosol koos 2 mmol/l kaaliumiga hemofiltratsioonilahus
Finland: Duosol cum 2 mmol/l Kalium hemofiltraationeste
France: Subsol avec 2 mmol/l potassium, solution pour hémofiltration hémodialyse et hémodiafiltration
Greece: Duosol without Potassium δι?λυμα αιμοδιαδι?θησης
Hungary: Nefrosol 2 mmol/l kálium hemofiltrációs oldat
Ireland: Nefrosol with 2 mmol/l Potassium solution for haemofiltration
Italy: Duosol con 2 mmol/l di potassio soluzione per emofiltrazione
Latvia: Duosol ar 2 mmol/l kalija škidums hemofiltracijai
Lithuania: Duosol K 2 hemofiltracijos tirpalas
Poland: Duosol zawierajacy 2 mmol/l potasu
Portugal: Duosol com potássio 2 mmol/l, solução para hemofiltração
Romania: Nefrosol cu 2 mmol/l potasiu, solutie pentru hemofiltrare
Slovenia: Duosol z 2 mmol/l kalija raztopina za hemofiltracijo
Spain: Priosol con 2 mmol/l de Potasio solución para hemofiltración
Netherlands: Duosol met 2 mmol/l Kalium, oplossing voor hemofiltratie
United Kingdom: Duosol with 2 mmol/l Potassium solution for haemofiltration
Date of last revision of this leaflet: September 2018
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Instructions for preparation of the ready-to-use haemofiltration solution
The packaging and solution must be visually inspected before use. Only haemofiltration solutions should be used if the packaging (outer wrapping and two-chamber bag), the peel seal, and the connectors are not damaged and are intact, and if the solution is clear and colourless and free from visible particles.
Remove the outer wrapping only immediately before use.
|
|
|
|
|
|
|
|
Administration of the ready-to-use haemofiltration solution
The haemofiltration solution should be warmed to approximately body temperature using an integrated or external warmer. The solution should not be perfused under any circumstances if it is below ambient temperature.
During the application of this medicine, precipitation of calcium carbonate in the tubes has been observed in rare cases, particularly near the pump unit and the heating unit. Therefore, during haemofiltration, the tubes should be visually inspected every 30 minutes to ensure that the solution in the tube system is clear and free from precipitates. Precipitation may also occur with a considerable delay after starting treatment. If precipitates are observed, the tubes should be replaced immediately and the patient should be closely monitored.
For single use only. Any remaining fraction of the solution and any damaged packaging should be discarded.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRIOSOL CON 2 mmol/L POTASSIUM HEMOFILTRATION SOLUTIONDosage form: HEMOFILTRATION, 2 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, 4 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, -Active substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription required
Online doctors for PRIOSOL CON 2 mmol/L POTASSIUM HEMOFILTRATION SOLUTION
Discuss questions about PRIOSOL CON 2 mmol/L POTASSIUM HEMOFILTRATION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions