

Biphozil

Ask a doctor about a prescription for Biphozil

How to use Biphozil
Leaflet accompanying the packaging: information for the user
Biphozyl, solution for hemodialysis/hemofiltration
Composite product
You should carefully read the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor, pharmacist, or nurse.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Biphozyl and what is it used for
- 2. Important information before using Biphozyl
- 3. How to use Biphozyl
- 4. Possible side effects
- 5. How to store Biphozyl
- 6. Contents of the packaging and other information
1. What is Biphozyl and what is it used for
Biphozyl is a solution for dialysis therapy (hemofiltration, hemodialysis, and hemodiafiltration) used to remove waste products from the blood when the kidneys are not working properly. The medicine is used in a hospital as part of intensive therapy using continuous renal replacement therapy (CRRT). In particular, the medicine is used in critically ill patients with acute kidney injury, in whom:
- the potassium level in the blood is normal,
- the blood pH value is normal,
- the phosphate level in the blood is normal,
- the calcium level in the blood is high (hypercalcemia).
This medicine may also be used:
- when other sources of bicarbonate are available or when the extracorporeal circuit is anticoagulated with citrate,
- in cases of poisoning with drugs or substances that are dialyzable or filterable.
2. Important information before using Biphozyl
Biphozyl should not be used in the following cases:
- hypersensitivity to one of the active substances or to any of the other ingredients of this medicine (listed in section 6),
- low calcium level in the blood (hypocalcemia),
- high potassium level in the blood (hyperkalemia),
- high phosphate level in the blood (hyperphosphatemia).
1/8
Warnings and precautions
Warnings
Before starting treatment with Biphozyl, you should discuss it with your doctor, pharmacist, or nurse.
Biphozyl should not be used in patients with high potassium levels in the blood. The potassium level in the patient's blood will be regularly monitored before and during treatment.
Since Biphozyl contains potassium, after starting treatment, a temporary high potassium level in the blood may occur. The doctor will reduce the infusion rate until the proper potassium level is achieved. If the normal state is not restored, the doctor will have to stop the administration of the medicine immediately. A potassium-free solution may be temporarily administered to restore the proper potassium level.
Since Biphozyl contains phosphates, after starting treatment, a temporary high phosphate level in the blood may occur. The doctor will reduce the infusion rate until the proper phosphate level is achieved. If the normal state is not restored, the doctor will have to stop the administration of the medicine immediately.
Since Biphozyl does not contain glucose, during treatment, a low glucose level in the blood may occur. The glucose level in the blood will be regularly monitored. If a low glucose level in the blood occurs, the doctor may administer a glucose-containing solution. Other measures may also be necessary to maintain the proper glucose level in the blood.
In patients receiving Biphozyl, the doctor will regularly monitor the hemodynamic status, fluid balance, and electrolyte and acid-base balance, including the volumes of fluids administered (intravenous infusions) and removed (urine output), even those not directly related to CRRT.
The medicine contains hydrogen phosphate, a weak acid that can affect the patient's acid-base balance. If, during therapy with Biphozyl, the bicarbonate level in the serum decreases or worsens, the doctor will reduce the infusion rate. If the normal state is not restored, the doctor will have to stop the administration of the medicine immediately.
You should strictly follow the instructions for use.
Before use, you should mix the solutions contained in the two chambers.
Use only with a dialysis machine for continuous renal replacement therapy (CRRT).
Use only if the outer packaging and the solution bag are not damaged. All seams must be intact. Using a contaminated solution can cause sepsis and shock.
You should only use it with suitable extracorporeal devices for extrarenal exchange.
Precautions
This medicine does not contain calcium and may cause hypocalcemia. Calcium infusion may be necessary.
To increase patient comfort, Biphozyl can be warmed to a temperature of +37°C. Warming the solution before use should be done before reconstitution and only using a dry heat source. Solutions should not be heated in water or a microwave oven. Before administration, you should visually inspect Biphozyl for the presence of solid particles and color change. Do not administer if the solution is not clear and the seam is damaged.
2/8
During the procedure, the doctor will closely monitor the patient's hemodynamic status, fluid balance, and electrolyte and acid-base balance, including the volumes of fluids administered (intravenous infusions) and removed (urine output), even those not directly related to CRRT.
The medicine contains bicarbonate in an amount corresponding to the lower limit of the normal concentration range in the blood. This is an amount suitable for use with citrate anticoagulation, as citrate is metabolized to bicarbonate, or in case of restoration of normal pH. An assessment of the need for buffering substances is necessary based on repeated measurements of blood acid-base balance and overall analysis of therapy. It may be necessary to use a solution with a higher bicarbonate content.
In case of excessive fluid volume in the body (hypervolemia), the ultrafiltration rate (net) in the CRRT device can be increased and/or the infusion rate of fluids other than substitution fluid and/or dialysate can be reduced.
In case of too little fluid volume in the body (hypovolemia), the ultrafiltration rate (net) in the CRRT device can be reduced and/or the infusion rate of fluids other than substitution fluid and/or dialysate can be increased.
Children
No special precautions are foreseen in the use of this medicine in children.
Elderly patients
No special precautions are foreseen in the use of this medicine in elderly patients.
Biphozyl and other medicines
You should tell your doctor, pharmacist, or nurse about all medicines you are taking or have recently taken, as well as any medicines you plan to take. This includes medicines obtained without a prescription. This is because the levels of other medicines in the blood may be reduced due to removal by dialysis. The doctor will decide whether any changes to the dosing of your medicines are necessary.
In particular, you should tell your doctor about the use of:
- additional sources of phosphates (e.g., parenteral nutrition fluids), as they may increase the risk of high phosphate levels in the blood (hyperphosphatemia).
- sodium bicarbonate, as it may increase the risk of excessive bicarbonate levels in the patient's blood (metabolic alkalosis).
- citrate as an anticoagulant, as it may reduce the calcium level in the serum.
Pregnancy, breastfeeding, and fertility
Pregnancy and breastfeeding:
There are no clinical data available on the use of this medicine during pregnancy and lactation. This medicine should only be administered to pregnant and breastfeeding women if clearly necessary.
Fertility:
No effect on fertility is anticipated, as sodium, potassium, magnesium, chloride, phosphate, and bicarbonate are normal components of the body.
Driving and using machines
It is not known whether this medicine affects the ability to drive and use machines.
3/8
3. How to use Biphozyl
Intravenous administration and use in hemodialysis. This medicine is intended for use in hospitals and should only be administered by healthcare professionals. The volume and rate of administration of the medicine depend on the patient's condition. The volume of the dose will be determined by the doctor.
This medicine should always be used exactly as prescribed by your doctor, pharmacist, or nurse. In case of doubt, you should consult your doctor, pharmacist, or nurse.
It is the responsibility of the doctor to determine the compatibility of additional medicines with this medicine by checking the solution for possible color change and/or precipitation. Before adding another medicine, you should check its solubility and stability in this medicine.
Dosage
Range of flow rates when used as a substitution fluid in hemofiltration and hemodiafiltration:
Adults:
500–3000 ml/hour.
Children under 18 years:
1000 to 4000 ml/hour/1.73 m
Range of flow rates when used as dialysate in continuous hemodialysis and continuous hemodiafiltration:
Adults:
500–2500 ml/hour.
Children under 18 years:
1000 to 4000 ml/hour/1.73 m
In the case of adolescents (12–18 years), if the calculated dose for children exceeds the maximum dose for adults, the dose recommended for adults should be used.
Instructions for use
This medicine will be administered to you in a hospital. Your doctor knows how to administer the medicine. The instructions for use are included at the end of this leaflet.
Using a higher dose of Biphozyl than recommended
If you feel unwell after taking a dose of the medicine higher than recommended in this leaflet or prescribed by your doctor, you should immediately consult your doctor or nurse.
Symptoms of overdose are fatigue, edema, or dyspnea.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Your doctor or nurse will regularly perform blood tests and monitor the clinical condition of patients to detect any possible side effects. The use of this solution may cause the following side effects (with unknown frequency, i.e., frequency cannot be estimated from the available data):
- changes in electrolyte levels in the blood (electrolyte imbalance), such as low calcium levels (hypocalcemia), high potassium levels (hyperkalemia), and high phosphate levels (hyperphosphatemia);
- decreased bicarbonate levels in the serum (metabolic acidosis).
Dialysis therapies can also cause certain side effects, such as:
- excessive (hypervolemia) or insufficient (hypovolemia) fluid volume in the body;
- decreased blood pressure;
4/8
- nausea, vomiting;
- muscle cramps.
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, you should tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Biphozyl
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label and packaging.
The expiry date refers to the last day of the stated month.
There are no special precautions for storage.
Do not freeze.
It has been demonstrated that during use, the solution after reconstitution remains chemically and physically stable for 24 hours at a temperature of +22°C. If the solution is not used immediately, the user is responsible for determining the storage conditions and time before use; however, the storage time should not exceed 24 hours, including the duration of treatment.
The solution can be disposed of in the sewage system without harm to the environment.
Do not use this medicine if you notice damage to the product or the presence of solid particles in the solution. All seams must be intact.
6. Contents of the packaging and other information
What Biphozyl contains
Before reconstitution
Small chamber A (250 ml):
Magnesium chloride hexahydrate
3.05 g/l
Large chamber B (4750 ml):
Sodium chloride
7.01 g/l
Sodium bicarbonate
2.12 g/l
Potassium chloride
0.314 g/l
Disodium phosphate dihydrate
0.187 g/l
5/8
After reconstitution
ready-to-use solution, A+B:
Active substances mmol/l mEq/l
Sodium ions, Na
140
140
Potassium ions, K
4
4
Magnesium ions, Mg
0.75
1.5
Chloride ions, Cl
122
122
Phosphate ions, HPO
1
2
Bicarbonate ions, HCO
22
22
Theoretical osmolality: 290 mOsm/l
pH = 7.0–8.0
Other ingredients are:
Hydrochloric acid diluted (for pH adjustment) E 507
Water for injections
Carbon dioxide (for pH adjustment) E 290
What Biphozyl looks like and what the packaging contains
This medicine is a solution for hemodialysis/hemofiltration packaged in a dual-chamber bag made of a multi-layered film containing polyolefins and elastomers. The final solution is obtained after opening the breakable seam and mixing the solutions contained in the large and small chambers. The solution is clear and colorless.
Each bag contains 5000 ml of solution and is wrapped in a transparent film.
Each box contains two bags and one patient leaflet.
Marketing authorization holder
Vantive Belgium SRL
Boulevard d’Angleterre 2
1420 Braine-l’Alleud
Belgium
Manufacturer
Bieffe Medital S.p.A.
Via Stelvio, 94
23035 Sondalo (SO)
Italy
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom (Northern Ireland): BIPHOZYL
Bulgaria: BIPHOZYL (Бифозил)
Date of last revision of the leaflet: September 2024 ---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Dosage
The volume and rate of administration of Biphozyl depend on the phosphate and other electrolyte levels in the blood, acid-base balance, fluid balance, and overall clinical condition of the patient. The volume of the solution to be administered will also depend on the desired intensity of treatment (dose).
The method of administration (dose, infusion rate, and total volume) of Biphozyl should be determined only by a doctor with experience in intensive therapy and continuous renal replacement therapy (CRRT).
Range of flow rates when used as a substitution fluid in hemofiltration and hemodiafiltration:
Adults:
500–3000 ml/hour.
Range of flow rates when used as dialysate in continuous hemodialysis and continuous hemodiafiltration:
Adults:
500–2500 ml/hour.
In adults, a combined flow rate during CRRT (dialysate and substitution solutions) of approximately 2000 to 2500 ml/hour is often used, which corresponds to a daily fluid volume of approximately 48 to 60 liters.
Children and adolescents
In children, from newborns to adolescents up to 18 years, the range of flow rates during use as a substitution fluid in hemofiltration and hemodiafiltration and as a dialysate in continuous hemodialysis and continuous hemodiafiltration is from 1000 to 4000 ml/hour/1.73 m.
In the case of adolescents (12–18 years), if the calculated dose for children exceeds the maximum dose for adults, the dose recommended for adults should be used.
Elderly patients
Adults over 65 years: clinical trial data and clinical experience indicate that the use of the product in elderly patients does not affect safety or efficacy.
Overdose
Symptoms of overdose
Overdose of Biphozyl may lead to severe clinical changes, such as congestive heart failure, electrolyte disturbances, or acid-base disturbances.
Procedure in case of overdose
- Hypervolemia/hypovolemia In case of hypervolemia or hypovolemia, you should strictly follow the instructions provided in section 2 Warnings.
- Metabolic acidosis If overdose results in metabolic acidosis and/or hyperphosphatemia, you should immediately stop administering the product. There is no specific antidote for overdose of the product. However, the risk can be minimized by close monitoring during treatment.
Preparation and/or use
The solution from the small chamber is added to the solution from the large chamber by opening the breakable seam directly before use. The solution after reconstitution should be clear and colorless.
In the entire process of administering the product to the patient, aseptic technique should be used.
Use only if the outer protective packaging is not damaged, all seams are intact, the breakable seam is not damaged, and the solution is clear. Firmly squeeze the bag to check its integrity. If a leak is detected, the solution should be discarded immediately, as sterility cannot be guaranteed.
The large chamber has a port for injection, which allows the addition of other necessary medicinal products after reconstitution of the solution. The user is responsible for determining the compatibility of the added medicinal product with Biphozyl by checking for possible color change and/or precipitation, presence of insoluble complexes or crystals. Before adding the medicinal product, you should check if it is soluble and stable in Biphozyl and if the pH range of Biphozyl is suitable for it (the pH of the solution after reconstitution is between 7.0-8.0). Additional ingredients may not be compatible with the solution. You should read the instructions for use of the added medicinal product.
After adding the ingredients, the solution should be thoroughly mixed. The addition and mixing of ingredients should always be done before connecting the bag with the solution to the extracorporeal circuit.
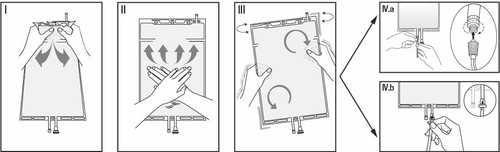
I
Open the seam by holding the small chamber with both hands and squeezing until a gap is created in the breakable seam between the two chambers (see figure I below).
II
Squeeze the large chamber with both hands until the breakable seam between the two chambers is completely open (see figure II below).
III
Mix the solution thoroughly by gently shaking the bag. The solution is ready for use, and the bag can be hung on the stand of the device (see figure III below).
IV
The dialysate line drain or the substitution fluid line drain can be connected to one of the two access ports.
IV.aIn the case of luer-type connectors, you should remove the cap by twisting and pulling it, and then connect the male luer lock connector on the dialysate line drain or the substitution fluid line drain to the female luer connector on the bag by pushing and twisting.
Make sure the connectors are fully seated, then tighten. The connector is now open.
Make sure the fluid flows freely (see figure IV.a below).
When the dialysate line drain or the substitution fluid line drain is disconnected from the luer connector, the connector will close, stopping the flow of the solution. The luer connector is needle-free and can be wiped with disinfectants.
IV.bIn the case of an injection connector (or a spike connector), you should first remove the sliding cap. The injection port can be wiped with disinfectants. Insert the spike through the rubber septum. Make sure the fluid flows freely (see figure IV.b below).
The solution after reconstitution is intended for single use only. Unused solution should be discarded. The solution can be disposed of in the sewage system without harm to the environment.
8/8
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterBieffe Medital S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BiphozilDosage form: Solution, 9 mg/mlActive substance: sodium chloridePrescription requiredDosage form: Solution, 100 mg/mlActive substance: dextranManufacturer: Fresenius Kabi Italia S.r.L.Prescription not requiredDosage form: Concentrate, -Active substance: electrolytes in combination with other drugsManufacturer: Fresenius Kabi Norge ASPrescription not required
Alternatives to Biphozil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Biphozil in Ukraine
Alternative to Biphozil in Spain
Online doctors for Biphozil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Biphozil – subject to medical assessment and local rules.














