PREVENAR 20 INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE

How to use PREVENAR 20 INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Prevenar 20 Injectable Suspension
Pneumococcal conjugate polysaccharide vaccine (20-valent, adsorbed)
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you or your child receive this vaccine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you or your child, do not pass it on to others.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Prevenar 20 and what is it used for
- What you need to know before you or your child receive Prevenar 20
- How Prevenar 20 is administered
- Possible side effects
- Storage of Prevenar 20
- Contents of the pack and further information
1. What is Prevenar 20 and what is it used for
Prevenar 20 is a pneumococcal vaccine administered to:
- Children from 6 weeks to less than 18 years of ageto help prevent diseases such as: meningitis (inflammation around the brain), sepsis or bacteremia (bacteria in the bloodstream), pneumonia (lung infection), and ear infections (acute otitis media) caused by 20 types of the Streptococcus pneumoniaebacteria.
- Individuals from 18 years of age and olderto help prevent diseases such as: pneumonia (lung infection), sepsis or bacteremia (bacteria in the bloodstream), and meningitis (inflammation around the brain) caused by 20 types of the Streptococcus pneumoniaebacteria.
Prevenar 20 provides protection against 20 types of the Streptococcus pneumoniaebacteria.
The vaccine works by helping the body to produce its own antibodies, which protect you or your child against these diseases.
2. What you need to know before you or your child receive Prevenar 20
Prevenar 20 must not be administered
- if you or your child are allergic (hypersensitive) to the active substances or to any of the other components of this medicine (listed in section 6) or to any other vaccine containing diphtheria toxoid.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before vaccination if you or your child:
- Have had any medical problems after receiving any dose of Prevenar 20, such as an allergic reaction or breathing problems.
- Have a severe illness or high fever. However, a mild fever or upper respiratory tract infection (such as a cold) is not a reason to delay vaccination.
- Have bleeding problems or bruise easily.
- Have a weakened immune system (e.g., due to HIV infection); you or your child may not get the full benefit of Prevenar 20.
Talk to your doctor, pharmacist, or nurse before vaccination if your child was born very prematurely (at 28 weeks of gestation or earlier), as longer pauses between breaths than normal may occur for 2 or 3 days after vaccination.
As with any vaccine, Prevenar 20 will not protect all vaccinated individuals.
Prevenar 20 will only protect against ear infections caused by the types of Streptococcus pneumoniaefor which the vaccine was developed. It will not protect against other infectious agents that may cause ear infections.
Other medicines/vaccines and Prevenar 20
Your child can receive Prevenar 20 at the same time as other pediatric scheduled vaccines.
In adults, Prevenar 20 can be administered at the same time as the flu vaccine (inactivated flu virus) in different injection sites. According to individual risk assessment by your healthcare professional, it may be recommended to separate the administration of both vaccines by, e.g., 4 weeks.
In adults, Prevenar 20 can be administered at the same time as the COVID-19 mRNA vaccine.
Tell your doctor, pharmacist, or nurse if you or your child are taking, have recently taken, or might take any other medicines or have recently received any other vaccine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before receiving this vaccine.
Driving and using machines
Prevenar 20 has no or negligible influence on the ability to drive and use machines. However, some of the effects mentioned in section 4 "Possible side effects" may temporarily affect the ability to drive or use machines.
Prevenar 20 contains polysorbate 80
This vaccine contains 0.1 mg of polysorbate 80 per dose. Polysorbates may cause allergic reactions. Inform your doctor if you or your child have any known allergy.
Prevenar 20 contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; i.e., it is essentially "sodium-free".
3. How Prevenar 20 is administered
Your doctor or nurse will inject the recommended dose (0.5 ml) of the vaccine into your child's arm or thigh.
Infants from 6 weeks to 15 months of age
Your child should receive an initial series of three injections of the vaccine followed by a booster dose.
- The first injection can be given from 6 weeks to 8 weeks of age.
- Each injection will be given separately with an interval of at least 4 weeks between doses, except for the last injection (booster dose), which will be given between 11 and 15 months of age.
You will be informed when your child should come back for the next injections.
According to the official recommendations of your country, consult your doctor, pharmacist, or nurse for more information.
Preterm infants (born before 37 weeks of gestation)
Your child will receive an initial series of three injections followed by a booster dose. The first injection can be given at 6 weeks of age with at least 4 weeks between doses. Between 11 and 15 months of age, your child will receive a fourth injection (booster dose).
Infants from 7 months to less than 12 months of age not previously vaccinated
Infants from 7 months to less than 12 monthsof age should receive three injections. The first two injections will be given with an interval of at least 4 weeks. A third injection will be given in the second year of life.
Children from 12 months to less than 24 months of age not previously vaccinated
Children from 12 months to less than 24 monthsof age should receive two injections, with at least 8 weeks between them.
Children from 2 years to less than 5 years of age not previously vaccinated
Children from 2 years to less than 5 yearsof age should receive one injection.
Children from 15 months to less than 5 years of age with previous complete vaccination with Prevenar 13
Children from 15 months to less than 5 yearsof age with previous complete vaccination with Prevenar 13 will receive one injection.
Children and adolescents from 5 years to less than 18 years of age regardless of previous vaccination with Prevenar 13
Children and adolescents from 5 years to less than 18 yearsof age will receive one injection.
If your child has received Prevenar 13 previously, an interval of at least 8 weeks should pass before receiving Prevenar 20.
Adults
Adults should receive one injection.
Tell your doctor, pharmacist, or nurse if you have been given a pneumococcal vaccine previously.
If you have any other questions about the use of Prevenar 20, ask your doctor, pharmacist, or nurse.
Special populations
Individuals at higher risk of pneumococcal infection (such as those with sickle cell disease or HIV infection), including those previously vaccinated with the 23-valent pneumococcal polysaccharide vaccine, may receive at least one dose of Prevenar 20.
Individuals with hematopoietic stem cell transplants may receive three injections, the first between 3 and 6 months after the transplant and with an interval of at least 4 weeks between doses. A fourth injection (booster dose) is recommended 6 months after the third injection.
4. Possible side effects
Like all vaccines, Prevenar 20 can cause side effects, although not everybody gets them.
Serious side effects of Prevenar 20
Tell your doctor immediately if you notice signs of the following serious side effects (see also section 2): swelling of the face, lips, mouth, tongue, or throat (edema), difficulty breathing (dyspnea), wheezing (bronchospasm) - these may be signs of a severe allergic reaction such as anaphylaxis, including anaphylactic shock.
Other side effects
The following side effects include those reported for Prevenar 20 in infants and children (from 6 weeks to less than 5 years of age):
Very common:may occur with more than 1 in 10 doses of the vaccine
- Decreased appetite
- Irritability
- Sleepiness
- Fever
- At the injection site for all children: redness, hardness, or swelling, pain or tenderness
- At the injection site after the booster dose and in children from 2 to less than 5 years of age: redness, hardness, or swelling of more than 2.0 to 7.0 cm
Common:may occur with up to 1 in 10 doses of the vaccine
- Diarrhea
- Vomiting
- Rash
- Fever (high temperature of 38.9°C or higher)
- At the injection site after the initial series of injections: redness, hardness, swelling of more than 2.0 to 7.0 cm, pain or tenderness that interferes with movement.
Uncommon:may occur with up to 1 in 100 doses of the vaccine
- Seizures (or fits), including those caused by a high temperature
- Hives (urticaria or urticaria-like rash)
- At the injection site: redness, hardness, or swelling of more than 7.0 cm
Rare:may occur with up to 1 in 1,000 doses of the vaccine
- Allergic reaction (hypersensitivity) at the injection site
The following side effects were observed with Prevenar 13 and may also be observed with Prevenar 20:
- Collapsing or shock-like state (hypotonic-hyporesponsive episode)
- Allergic reaction (hypersensitivity) including swelling of the face or lips
- Crying
- Restless sleep
The following side effects include those reported for Prevenar 20 in children and adolescents (5 years to less than 18 years of age):
Very common:may occur with more than 1 in 10 doses of the vaccine
- Headache
- Muscle pain
- Pain or tenderness at the injection site and fatigue
Common:may occur with up to 1 in 10 doses of the vaccine
- Swelling at the injection site, redness at the injection site, and fever
Uncommon:may occur with up to 1 in 100 doses of the vaccine
- Hives (urticaria or urticaria-like rash)
- Fever
The following side effects were observed with Prevenar 13 and may also be observed with Prevenar 20:
- Diarrhea
- Vomiting
- Decreased appetite
- Irritability
- Sleepiness
- Restless sleep
- Rash
Children and adolescents with HIV infection, sickle cell disease, or hematopoietic stem cell transplants had similar side effects; however, the frequencies of vomiting, diarrhea, fever, joint pain, and injection site pain or tenderness that interferes with movement were very common.
The following side effects were observed with Prevenar 13 in the post-marketing experience in children and may also be observed with Prevenar 20:
- Severe allergic reaction including anaphylactic shock; swelling of the lips, face, or throat (angioedema)
- Enlarged lymph nodes or glands (lymphadenopathy) near the injection site, such as under the arm or in the groin
- At the injection site: hives (urticaria), redness, and irritation (dermatitis), and itching (pruritus)
- A rash that causes red spots (erythema multiforme)
The following side effects include those reported for Prevenar 20 in adults:
Very common:may occur with more than 1 in 10 doses of the vaccine
- Headache
- Joint pain and muscle pain
- Pain/sensitivity at the injection site and fatigue
Common:may occur with up to 1 in 10 doses of the vaccine
- Swelling at the injection site, redness at the injection site, and fever
Uncommon:may occur with up to 1 in 100 doses of the vaccine
- Diarrhea, nausea, and vomiting
- Rash and swelling of the face, lips, mouth, tongue, or throat that may cause difficulty swallowing or breathing (angioedema)
- Itching at the injection site, inflammation of the lymph nodes in the neck, armpits, or groin (lymphadenopathy), hives at the injection site (urticaria), and chills
The following side effects were observed with Prevenar 13 and may also be observed with Prevenar 20:
- A rash that causes red spots (erythema multiforme)
- Irritation at the injection site
- Decreased appetite
- Limited movement of the arm
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Prevenar 20
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after "EXP". The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Prevenar 20 should be used as soon as possible after removal from the refrigerator.
Do not freeze. Discard if the vaccine has been frozen.
Stability data indicate that the vaccine is stable for 96 hours when stored at temperatures between 8°C and 25°C or 72 hours when stored at temperatures between 0°C and 2°C. At the end of these time periods, Prevenar 20 should be used or discarded. These data are intended to guide healthcare professionals in case of temporary temperature variations only.
Pre-filled syringes should be stored horizontally to minimize resuspension time.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Prevenar 20 composition
The active ingredients are polysaccharide conjugates with CRM197 consisting of:
- 2.2 micrograms of polysaccharide from serotypes 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F.
- 4.4 micrograms of polysaccharide from serotype 6B.
A dose (0.5 ml) contains approximately 51 micrograms of carrier protein CRM197, adsorbed on aluminum phosphate (0.125 mg of aluminum).
The other components are sodium chloride, succinic acid, polysorbate 80, and water for injectable preparations.
Appearance of Prevenar 20 and container contents
The vaccine is a white injectable suspension presented in a single-dose pre-filled syringe (0.5 ml). It is presented in packs of 1, 10, and 50, with or without needles. Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer responsible for batch release
Marketing authorization holder: Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Brussels Belgium | Manufacturer responsible for batch release: Pfizer Manufacturing Belgium NV Rijksweg 12 2870 Puurs-Sint-Amands Belgium |
Further information on this medicinal product can be obtained from the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: + 32 (0)2 554 62 11 | Latvija Pfizer Luxembourg SARL filiale Latvija Tel.: + 371 670 35 775 |
| Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. + 370 5 251 4000 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Magyarország Pfizer Kft Tel: + 36 1 488 37 00 |
Danmark Pfizer ApS Tlf.: + 45 44 20 11 00 | Malta Vivian Corporation Ltd. Tel: + 356 21344610 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Ελλáδα Pfizer Ελλáς A.E. Τηλ.: +30 210 6785800 | Österreich Pfizer Corporation Austria Ges.m.b.H Tel: +43 (0)1 521 15-0 |
España Pfizer, S.L. Télf: +34 91 490 99 00 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
France Pfizer Tél +33 (0)1 58 07 34 40 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | România Pfizer Romania S.R.L Tel: +40 (0) 21 207 28 00 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: +1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel.: +386 (0)1 52 11 400 |
Ísland Icepharma hf. Simi: + 354 540 8000 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Kúπρος Pfizer Ελλáς Α.Ε. (Cyprus Branch) Tηλ: +357 22817690 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Date of last revision of this leaflet: 04/2025.
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
This information is intended for healthcare professionals only:
During storage, a white sediment and a clear supernatant may be observed. This does not constitute a sign of deterioration. Pre-filled syringes should be stored horizontally to minimize resuspension time.
Preparation for administration
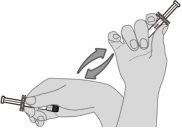
Step 1. Resuspension of the vaccine Hold the pre-filled syringe horizontally between the thumb and index finger and shake vigorously until the syringe contents are a homogeneous white suspension. Do not use the vaccine if it cannot be resuspended. |
|
Step 2. Visual inspection Visually inspect the vaccine for large particles and color variations before administration. Do not use it if large particles or color variations are found. If the vaccine is not a homogeneous white suspension, repeat steps 1 and 2. |
|
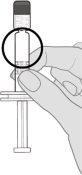
Step 3. Remove the syringe cap Remove the syringe cap from the Luer lock adapter by slowly turning the cap counterclockwise while holding the Luer lock adapter. Note: Care should be taken not to press the extended plunger while removing the syringe cap. |
|
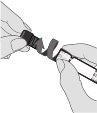
Step 4. Place a sterile needle
Place a suitable needle for intramuscular administration on the pre-filled syringe by holding the Luer lock adapter and turning the needle clockwise.
Administer the full dose.
Prevenar 20 is for intramuscular injection only.
Prevenar 20 should not be mixed with other vaccines or medicines in the same syringe.
Prevenar 20 can be administered at the same time as other pediatric vaccines; in this case, different injection sites should be used.
Prevenar 20 can be administered to adults at the same time as the seasonal influenza vaccine (QIV; surface antigen, inactivated, with adjuvant). In subjects with underlying conditions associated with a high risk of developing potentially fatal pneumococcal disease, it may be considered to separate the administrations of QIV and Prevenar 20 (e.g., approximately 4 weeks). Different injection sites should be used.
Prevenar 20 can be administered to adults at the same time as the COVID-19 mRNA vaccine (with modified nucleosides).
Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PREVENAR 20 INJECTABLE SUSPENSION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 4 micrograms/doseActive substance: pneumococcus, purified polysaccharides antigen conjugatedManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE, 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 4.4 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µgActive substance: pneumococcus, purified polysaccharides antigen conjugatedManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µg / 4.4 µg / 2.2 µg / 2.2 µg / 2.2 µg / 2.2 µgActive substance: pneumococcus, purified polysaccharides antigen conjugatedManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for PREVENAR 20 INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Discuss questions about PREVENAR 20 INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions