
OZALIN 2 mg/ml ORAL SOLUTION IN SINGLE-DOSE PACKAGES

How to use OZALIN 2 mg/ml ORAL SOLUTION IN SINGLE-DOSE PACKAGES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET
Package Leaflet: Information for the User
OZALIN2mg/ml oral solution in single-dose container
Midazolam
Read all of this leaflet carefully before your child starts using this medicine because it contains important information for them.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What OZALIN is and what it is used for
- What you need to know before your child is given OZALIN
- How to use OZALIN
- Possible side effects
- Storage of OZALIN
- Contents of the pack and further information
1. What OZALIN is and what it is used for
This medicine contains midazolam. It belongs to a group of medicines known as benzodiazepines.
This medicine is used in infants, children, and adolescents from 6 months to 17 years of age to provide moderate sedation:
- before a diagnostic or therapeutic procedure to relieve anxiety, discomfort, and agitation related to the procedure;
- as pre-anesthetic medication.
2. What you need to know before your child is given OZALIN
Do not use OZALIN
- if your child is allergic to midazolam, benzodiazepines, or any of the other ingredients of this medicine (listed in section 6);
- if your child has a severe neuromuscular disease that causes muscle weakness (myasthenia gravis);
- if your child has severe breathing difficulties;
- if your child has a disease that causes frequent interruption of breathing during sleep (sleep apnea syndrome);
- if your child has severe liver problems.
Warnings and precautions
Talk to your doctor or pharmacist before your child is given OZALIN
- if your child has a long-term disease (such as respiratory, kidney, liver, or heart problems);
- if your child is in poor general health;
- if your child has a history of alcoholism or drug addiction;
- if your child is under 6 months old.
Other medicines and OZALIN
Tell your doctor or pharmacist if your child is taking, has recently taken, or might take any other medicines, and in particular if your child is taking any of the following medicines:
- medicines used to treat bacterial infections (antibiotics), e.g., erythromycin, clarithromycin, telithromycin, roxithromycin;
- medicines used to treat fungal infections (antifungals), e.g., ketoconazole, voriconazole, fluconazole, itraconazole, and posaconazole;
- medicines used to treat stomach ulcers (anti-ulcer medicines), e.g., cimetidine, ranitidine;
- medicines used to treat epilepsy (antiepileptics), e.g., carbamazepine and phenytoin;
- medicines used to treat high blood pressure (antihypertensives), e.g., diltiazem and verapamil;
- medicines used to treat HIV and AIDS, e.g., saquinavir, including combinations with ritonavir and efavirenz;
- medicines used to prevent nausea and vomiting, e.g., aprepitant;
- medicines used to reduce fat in the blood, e.g., atorvastatin;
- medicines used to treat depression that cause drowsiness (sedative antidepressants);
- other medicines for treating depression (antidepressants), e.g., fluvoxamine;
- medicines used to treat cystic fibrosis, e.g., ivacaftor;
- medicines used to treat urinary incontinence, e.g., propiverine;
- medicines used to treat mycobacterial infections such as tuberculosis, e.g., rifampicin;
- medicines used as anesthetics, e.g., inhaled anesthetics, propofol, ketamine, etomidate;
- medicines that induce sleep (hypnotics);
- medicines used as strong painkillers (narcotic analgesics); e.g., fentanyl;
- medicines to relieve cough (antitussives) or for the treatment of opioid dependence (substitution treatment) that contain opioids;
- medicines used to treat specific mental disorders, such as psychosis (antipsychotics);
- medicines that contain benzodiazepines used to treat anxiety or sleep disorders (benzodiazepines used as anxiolytics or hypnotics);
- medicines for treating allergies (antihistamines):
- herbal medicines, e.g., St. John's Wort, Echinacea purpurea, turmeric rhizome.
Using OZALIN with food, drinks, and alcohol
General fasting guidelines should be followed before sedation.
Your child should not drink alcohol while taking this medicine. Alcohol may increase the sedative effects of this medicine and cause excessive drowsiness.
Your child should not drink grapefruit juice while taking this medicine. Grapefruit juice may increase the sedative effects of this medicine and cause excessive drowsiness.
Pregnancy and breastfeeding
Pregnancy
If your daughter is pregnant or you think she may be pregnant, ask your doctor for advice before your child is given this medicine.
Breastfeeding
If your daughter is breastfeeding, she should be informed of the need to stop breastfeeding for 24 hours after the administration of midazolam, as midazolam passes into breast milk in small amounts.
Driving and using machines
This medicine may cause drowsiness, poor memory, or affect concentration and coordination. Your child should not drive vehicles, ride bicycles, or use tools or machines until they have fully recovered. Talk to your doctor if you need more information.
OZALIN contains sodium, ethanol, and cyclodextrin
This medicine contains less than 23 mg of sodium (1 mmol) per ampoule; this is essentially "sodium-free".
This medicine contains 17.4 mg of alcohol (ethanol) in each single dose, ampoule of 5 ml, which is equivalent to 3.5 mg/ml (ethanol/solution) or 0.32% p/v.
The amount of ethanol in 1 ampoule of 5 ml of this medicine (17.4 mg) is equivalent to 0.2 ml of wine. The amount of ethanol in 2 ampoules of 5 ml of this medicine (34.8 mg) is equivalent to 0.4 ml of wine, at the maximum dose of 20 mg of midazolam.
The small amount of alcohol in this medicine does not produce any noticeable effect.
This medicine contains 400 mg of cyclodextrin in each ampoule, equivalent to 10 mg/kg/day at the recommended dose, and is below the permitted daily exposure. Therefore, even if OZALIN is accidentally used with doses of 0.5 mg/kg, the amount of cyclodextrin will not exceed the permitted daily exposure.
3. How to use OZALIN
Instructions for use
This medicine should be administered orally.
This medicine will be administered to your child by a healthcare professional. It will be administered in a place that has the necessary equipment to monitor your child and treat side effects.
This medicine is not intended for self-administration.
Your child should be accompanied by an adult at the time of discharge and should only leave the treatment room after receiving the doctor's authorization.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur when using midazolam. Their frequency cannot be determined from the available data.
Nervous system disorders:
- Prolonged sedation/oversedation;
- Agitation, restlessness, hostility, anger, or aggression, excitement, confusion, euphoria (an excessive feeling of happiness or excitement), or hallucinations (seeing and possibly hearing things that do not exist);
- Sleepiness, drowsiness;
- Dizziness;
- Difficulty coordinating muscles;
- Vertigo;
- Speech disorders;
- Dry mouth;
- Salivation;
- Urinary incontinence;
- Headache;
- Temporary memory loss;
Immune system disorders
- Hypersensitivity reactions and angioedema may occur in prone individuals.
- Chest pain has been observed as a sign of a severe allergic reaction called Kounis syndrome.
Cardiac disorders:
- Alteration of heart rate (slow or fast heart rate).
Respiratory disorders:
- Laryngospasm (narrowing of the vocal cords that causes difficulty breathing and noisy breathing), breathing difficulties (slow breathing), wheezing;
- Noisy breathing;
- Hiccup.
Gastrointestinal disorders:
- Vomiting;
- Nausea.
Eyeball disorders:
- Blurred vision;
- Double vision.
Skin disorders:
- Itching, skin rash with red lumps with itching (urticaria reaction);
- Skin rash.
General disorders and administration site conditions:
- Unusual tiredness;
- Feeling of weakness.
Reporting of side effects
If your child experiences side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system.
Spanish Medicines Agency: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of OZALIN
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the ampoule label, blister, or carton after CAD or EXP. The expiry date is the last day of the month stated.
Store in the original packaging to protect from light. Do not store above 25°C. Do not freeze or refrigerate.
6. Container Content and Additional Information
Composition ofOZALIN
- The active ingredient is midazolam.
- The other components are: citric acid monohydrate, gammadex, sucralose, orange flavor (contains mainly ethanol at 70-80%), sodium hydroxide (for pH adjustment), water for injectable preparations.
Appearance of OZALIN and Container Content
This medication is presented in the form of a 5 ml amber glass ampoule, a tube with a filter, and an oral applicator packaged in an individual blister pack.
This medication is marketed in 3 different presentations:
- blister pack of one
- blister pack of five
- blister pack of 10
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
ISTITUTO GENTILI S.R.L.
Via San Giuseppe Cottolengo 15
20143 Milan
Italy
Manufacturer
VALDEPHARM
Parc Industriel d’Incarville, 27106 Val-De-Reuil, France
Local Representative
QUALITECFARMA® S.L.
Paseo Del Pintor Rosales 42 Piso 3º A
28008 Madrid, Spain
This medication has been authorized in the EEA Member States under the following names:
Austria OZASED 2 mg/ml Lösung zum Einnehmen im Einzeldosisbehältnis
Belgium Ozalin 2 mg/ml solution buvable en récipient unidose / drank in verpakking voor éénmalig gebruik / Lösung zum Einnehmen im Einzeldosisbehältnis
Denmark Ozalin 2 mg/ml oral opløsning i enkeltdosisbeholder
Finland Ozalin 2 mg/ml oraaliliuos kerta-annospakkaus
France Ozalin 2 mg/ml solution buvable en récipient unidose
Germany Ozalin 2 mg/ml Lösung zum Einnehmen im Einzeldosisbehältnis
Greece Ozalin 2 mg/ml π?σιμο δι?λυμα σε περι?κτη μ?ας δ?σης
Ireland Ozalin 2 mg/ml oral solution in single-dose container
Italy Ozased 2 mg/ml soluzione orale in contenitore monodose
Norway Ozalin 2 mg/ml mikstur, oppløsning i endosebeholder
Poland OZASED, 2 mg/ml, roztwór doustny w pojemniku jednodawkowym
Portugal Ozalin 2 mg/ml solução oral em recipiente unidose
Spain Ozalin 2 mg/ml solución oral en envase unidosis
Netherlands Ozalin 2 mg/ml drank in verpakking voor eenmalig gebruik
Date of the Last Revision of this Leaflet:November 2022
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The solution should be inspected visually before use. Do not use this medication if you observe visible signs of deterioration in the solution or packaging. OZALIN should only be administered with its corresponding oral applicator graduated inkg.
How to Open the Ampoule
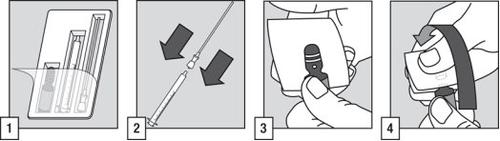
- The administration to the patient requires the use of the ampoule, the tube with a filter, and the oral applicator.
- Connect the tube with a filter to the end of the oral applicator.
- Touch the top of the ampoule to ensure that all the liquid has flowed to the bottom. Cover the top of the ampoule with a swab and place a thumb on the white dot.
- Hold the ampoule firmly with the white dot facing up and towards you. Press the neck of the ampoule and it will open easily.
Preparation and Administration of the Solution
- Insert the tube with a filter into the ampoule. Before adjusting the dose and to eliminate any possible air in the tube with a filter, a brief pumping with the applicator (filling and emptying) of the solution inside the ampoule is recommended.
- While holding the ampoule in a vertical position, fill the oral applicator up to the graduation mark corresponding to the patient's weight in kilograms (kg). Align the line mark with the top of the tab to take the correct dose.
- Remove the tube with a filter from the end of the oral applicator.
- Empty the contents of the oral applicator into the patient's mouth. The solution should be swallowed immediately.
- After use, discard the ampoule, the tube with a filter, the oral applicator, and any unused contents in a container prepared for this purpose in accordance with local requirements for controlled substances and pharmaceutical accessories.
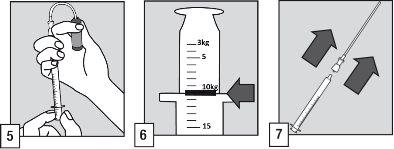
Dosage
The dose should be adapted to the patient's weight.
OZALIN should be used orally in a single dose of 0.25 mg/kg in children and adolescents from six months of age.
The maximum dose should not exceed 20 mg of midazolam (corresponding to 2 ampoules), even for children who weigh more than 80 kg.
In obese children and adolescents, the dose should be administered according to the actual body weight, up to the limit of 20 mg.
The oral applicator is graduated in kilograms, from3kg to40kg of body weight, with three types of graduation marks:
- A small graduation mark corresponding to 1 kg, i.e.,0.25 mg of midazolam;
- An intermediate graduation mark corresponding to 5 kg, i.e.,1.25 mg of midazolam;
- A large graduation mark corresponding to 10 kg, i.e.,2.50 mg of midazolam
For patients over 40 kg, 2 ampoules are needed. The minimum dose that should be obtained from an ampoule should correspond to a dose of 3 kg. For patients who weigh 41 and 42 kg who need more than one ampoule, a lower dose than that corresponding to 40 kg should be taken in the first ampoule and the supplement to the dose in the second ampoule, see examples below:
- For a patient of 41 kg, it is recommended to take a dose for 30 kg in the first ampoule and 11 kg in the second ampoule
- For a patient of 42 kg, take a dose corresponding to 30 kg in the first ampoule and 12 kg in the second ampoule.
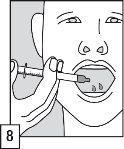
The oral applicator and the tube with a filter are single-use sampling and administration devices.
OZALIN should be administered within 30 minutes before the procedure or anesthesia.
OZALIN is not recommended in newborns (premature and full-term) and in infants under 6 months of age.
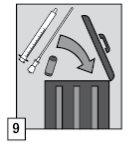
In case of overdose, vomiting should be induced (as soon as possible and in no case within one hour after oral administration of midazolam) if the patient is conscious, or gastric lavage should be performed while protecting the airways if the patient is unconscious. If gastric lavage is not effective, activated charcoal should be administered to reduce absorption.
Flumazenil, a benzodiazepine antagonist, is indicated in case of severe poisoning accompanied by respiratory depression or coma. This treatment should only be administered under close supervision and in accordance with local guidelines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OZALIN 2 mg/ml ORAL SOLUTION IN SINGLE-DOSE PACKAGESDosage form: GEL/PASTE/ORAL LIQUID, 10 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 2.5 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 5 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription required
Online doctors for OZALIN 2 mg/ml ORAL SOLUTION IN SINGLE-DOSE PACKAGES
Discuss questions about OZALIN 2 mg/ml ORAL SOLUTION IN SINGLE-DOSE PACKAGES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











