BUCCOLAM 2.5 mg ORAL SOLUTION


How to use BUCCOLAM 2.5 mg ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
BUCCOLAM 2.5mg buccal solution
For children from 3 months to less than 1 year
BUCCOLAM 5mg buccal solution
For children from 1 year to less than 5 years
BUCCOLAM 7.5mg buccal solution
For children from 5 years to less than 10 years
BUCCOLAM 10mg buccal solution
For children from 10 years to less than 18 years
Midazolam
Read the entire package leaflet carefully before starting to administer this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to your child, and you should not give it to others, even if they have the same symptoms as the child for whom it was prescribed, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is BUCCOLAM and what is it used for
- What you need to know before starting to administer BUCCOLAM
- How to administer BUCCOLAM
- Possible side effects
- Storage of BUCCOLAM
- Contents of the pack and further information
1. What is BUCCOLAM and what is it used for
BUCCOLAM contains a medicine called midazolam. Midazolam belongs to a group of medicines known as benzodiazepines. BUCCOLAM is used to stop a prolonged, acute seizure in infants, children, and adolescents (from 3 months to less than 18 years).
In infants from 3 months to less than 6 months, treatment should only be administered in a hospital where the patient can be monitored and equipped with resuscitation equipment.
This medicine should only be used by parents/caregivers when epilepsy has been diagnosed in the child.
2. What you need to know before starting to administer BUCCOLAM
Do not administer BUCCOLAM if the patient:
- is allergic to midazolam, benzodiazepines (such as diazepam) or any of the other ingredients of this medicine (listed in section 6);
- has a nerve and muscle disease that causes muscle weakness (myasthenia gravis);
- has serious breathing difficulties at rest (BUCCOLAM may worsen breathing difficulties);
- has a disease that causes frequent interruptions of breathing while sleeping (sleep apnea syndrome);
- has severe liver problems.
Warnings and precautions
Consult your doctor or pharmacist before starting to administer BUCCOLAM if the patient:
- has a kidney, liver, or heart condition;
- has a lung condition that causes periodic breathing difficulties.
This medicine may cause people to forget what happened after they were given the medicine. Patients should be closely observed after administration of this medicine.
This medicine should be avoided in patients with a history of alcoholism or drug abuse.
It is more likely that potentially fatal incidents will occur in patients with breathing difficulties or heart problems, especially when higher doses of BUCCOLAM are administered.
Children under 3 months: BUCCOLAM should not be administered to children under 3 months due to lack of information in this age group.
If you are unsure about any of the above, consult your doctor or pharmacist before administering this medicine.
Using BUCCOLAM with other medicines
Tell your doctor or pharmacist if the patient is using, has recently used, or might use any other medicines. If you are unsure about any medicine the patient is taking that may affect the use of BUCCOLAM, consult your doctor or pharmacist.
This is particularly important because using more than one medicine at the same time can increase or decrease the effects of the medicines taken.
The effects of BUCCOLAM may be increased by the following medicines:
- antiepileptics (for treating epilepsy), e.g., phenytoin
- antibiotics, e.g., erythromycin, clarithromycin
- antifungals, e.g., ketoconazole, voriconazole, fluconazole, itraconazole, posaconazole
- medicines for ulcers, e.g., cimetidine, ranitidine, and omeprazole
- medicines used to treat blood pressure, e.g., diltiazem, verapamil
- certain medicines used to treat HIV and AIDS, e.g., saquinavir, lopinavir/ritonavir combination
- narcotic painkillers (very strong pain relievers), e.g., fentanyl
- medicines used to reduce blood fat, e.g., atorvastatin
- medicines used to treat nausea, e.g., nabilone
- hypnotics (sleep-inducing medicines)
- sedating antidepressants (medicines for treating depression that cause sleepiness)
- sedatives (medicines to help relax)
- anesthetics (medicines to relieve pain)
- antihistamines (medicines for treating allergies)
The effects of BUCCOLAM may be reduced by the following medicines:
- rifampicin (used to treat tuberculosis)
- xanthines (used to treat asthma)
- St. John's Wort (a herbal medicine). This should be avoided in patients taking BUCCOLAM.
BUCCOLAM may increase the effect of some muscle relaxants, e.g., baclofen (causing increased sleepiness). This medicine may also cause some medicines to stop working as well, e.g., levodopa (a medicine used to treat Parkinson's disease).
Consult your doctor or pharmacist for more information about medicines that the patient should avoid while taking BUCCOLAM.
Using BUCCOLAM with food and drink
The patient should not drink alcohol while taking BUCCOLAM. Alcohol may increase the sedative effects of this medicine and cause excessive sleepiness.
The patient should not drink grapefruit juice while taking BUCCOLAM. Grapefruit juice may increase the sedative effects of this medicine and cause excessive sleepiness.
Pregnancy
If the patient who is to receive this medicine is pregnant or breastfeeding, thinks she may be pregnant, or plans to become pregnant, consult her doctor before using this medicine.
Administration of high doses of BUCCOLAM during the last 3 months of pregnancy may cause abnormal heart rhythm in the fetus. Children born after administration of this medicine during delivery may also experience difficulty feeding, breathing difficulties, and poor muscle tone at birth.
Breastfeeding
Tell your doctor if the patient is breastfeeding. Although small amounts of BUCCOLAM may pass into breast milk, it may not be necessary to stop breastfeeding. The doctor will advise whether the patient should breastfeed the baby after receiving this medicine.
Driving and using machines
BUCCOLAM may cause the patient to feel drowsy, forgetful, or affect concentration and coordination. This may interfere with performing tasks that require skill, such as driving, cycling, or using machines.
After receiving this medicine, the patient should not drive, cycle, or use machines until fully recovered. Ask your doctor if you need more information.
3. How to administer BUCCOLAM
Follow the administration instructions for this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
Dose
Your doctor will prescribe the correct dose of BUCCOLAM for your child, usually depending on the child's age. Each dose has a different color, which is shown on the box, tube, and syringe containing the medicine.
Your child will receive one of the following specific doses for their age in a color-coded pack:
3 months to less than 1 year: 2.5 mg – pack with yellow label
1 year to less than 5 years: 5 mg – pack with blue label
5 years to less than 10 years: 7.5 mg – pack with purple label
10 years to less than 18 years: 10 mg – pack with orange label
An oral syringe contains a complete dose. Do not administer more than one dose.
Infants from 3 months to less than 6 months should only receive treatment in a hospital where the patient can be monitored and equipped with resuscitation equipment.
Preparation for administration of this medicine
If the child has a seizure, let their body move freely; do not try to hold them. Only move them if they are in danger, for example, near deep water, fire, or sharp objects.
Support the child's head on a soft object, such as a cushion or your lap.
Check that the medicine contains the correct dose for your child, specific to their age.
How to administer this medicine
Ask a doctor, pharmacist, or nurse to show you how to take or administer this medicine. If you are unsure, always ask your doctor, pharmacist, or nurse.
Information on how to administer this medicine is also on the tube label.
BUCCOLAMshould not be injected. Do not put a needle in the syringe.
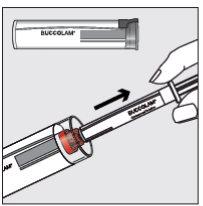
Step 1
| Hold the plastic tube, break the seal at one end, and remove the closure cap. Remove the syringe from the tube. |
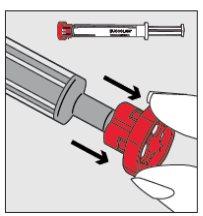
Step 2
| Remove the red closure cap from the syringe tip and dispose of it safely. |
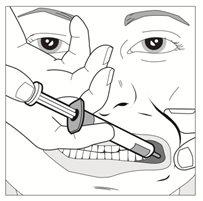
Step 3
| With the help of your index finger and thumb, gently pinch and pull back the child's cheek. Place the syringe tip in the back of the space between the inside of the cheek and the lower gum. |
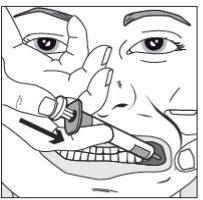
Step 4
| Slowly press the syringe plunger until it stops. Slowly introduce the entire solution into the space between the gum and the cheek (buccal cavity). If prescribed by your doctor (for larger volumes and/or smaller patients), you can administer half of the dose on one side of the mouth and then the other half on the other side of the child's mouth. |
When to call an ambulance Always follow the treatment recommendations provided by the patient's doctor or as indicated by the healthcare professional. If you are unsure, seek urgent medical help if:
Keep the syringe to show to the ambulance personnel or doctor. Do not administer more medicine than prescribed by the doctor for the patient. |
If the child vomits
- Do not administer another dose of BUCCOLAM to the patient.
- If the seizure does not stop within 10 minutes, call an ambulance.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Seek immediate medical attention or call for an ambulance if the patient experiences the following side effects:
- Severe breathing difficulties, e.g., slow or shallow breathing or blue lips. In very rare cases, breathing may stop.
- Heart attack. Signs may include chest pain radiating to the neck and shoulders and extending to the left arm.
- Swelling of the face, lips, tongue, or throat, making it difficult to swallow or breathe.
Other side effects
If the patient experiences any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet.
Common side effects (may affect up to 1 in 10 people):
- Nausea and vomiting
- Drowsiness or being less conscious
Uncommon side effects (may affect up to 1 in 100 people):
- Rash, hives (itchy rash), itching
Rare side effects (may affect up to 1 in 10,000 people):
- Agitation, restlessness, hostility, anger, or aggression, excitement, confusion, euphoria (excessive feeling of happiness or excitement), or hallucinations (seeing and possibly hearing things that are not really there)
- Muscle spasms and muscle tremors (trembling of muscles that cannot be controlled)
- Reduced alertness
- Headache
- Dizziness
- Difficulty coordinating muscles
- Seizures (convulsions)
- Transient loss of memory. The duration depends on the amount of BUCCOLAM administered.
- Low blood pressure, slow heart rate, or reddening of the face and neck (flushing)
- Laryngospasm (contraction of the vocal cords causing difficulty breathing and noise when breathing)
- Constipation
- Dry mouth
- Fatigue
- Hiccups
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of BUCCOLAM
Keep this medicine out of the sight and reach of children.
Do not administer this medicine after the expiry date stated on the carton and on the labels of the tube and oral syringe after EXP. The expiry date is the last day of the month shown.
Do not refrigerate or freeze.
Keep the oral syringe in the protective plastic tube.
Do not use this medicine if the pack is opened or damaged.
Disposal of oral syringes
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
BUCCOLAM composition
- The active ingredient is midazolam
- Each 2.5 mg oral pre-filled syringe contains 2.5 mg of midazolam (as hydrochloride) in 0.5 ml of solution.
- Each 5 mg oral pre-filled syringe contains 5 mg of midazolam (as hydrochloride) in 1 ml of solution.
- Each 7.5 mg oral pre-filled syringe contains 7.5 mg of midazolam (as hydrochloride) in 1.5 ml of solution.
- Each 10 mg oral pre-filled syringe contains 10 mg of midazolam (as hydrochloride) in 2 ml of solution.
The other ingredients are sodium chloride, water for injections, hydrochloric acid, and sodium hydroxide (for pH adjustment).
Product appearance and packaging contents
3 months to less than 1 year: 2.5 mg – packaging with yellow label
1 year to less than 5 years: 5 mg – packaging with blue label
5 years to less than 10 years: 7.5 mg – packaging with purple label
10 years to less than 18 years: 10 mg – packaging with orange label
BUCCOLAM oral solution is a clear, colorless liquid. It is supplied in a single-use, amber-colored oral pre-filled syringe. Each oral syringe is individually packaged in a protective plastic tube. BUCCOLAM is presented in boxes containing 4 oral pre-filled syringes/tubes (of the same dose).
Marketing authorization holder
Laboratorios Lesvi, S.L.
Avda. Barcelona, 69
08970 Sant Joan Despí - Barcelona
Spain
Tel: +34 93 602 24 21
E-mail: [email protected]
Manufacturer
Shire Pharmaceuticals Ireland Limited
Block 2 & 3 Miesian Plaza
50 – 58 Baggot Street Lower
Dublin 2
Ireland
Laboratorios Lesvi, S.L.
Avda. Barcelona, 69
08970 Sant Joan Despí - Barcelona
Spain
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium Neuraxpharm France Tel: +32 474 62 24 24 | Lithuania Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Bulgaria Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Luxembourg Neuraxpharm France Tel: +32 474 62 24 24 |
Czech Republic Neuraxpharm Bohemia s.r.o. Tel: +420 495 736 145 | Hungary Neuraxpharm Bohemia s.r.o. Tel: +36 (30) 542 2071 |
Denmark Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Malta Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Germany neuraxpharm Arzneimittel GmbH Tel: +49 2173 1060 0 | Netherlands Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Estonia Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Norway Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Greece Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Austria Neuraxpharm Austria GmbH Tel: +43 2236 389836 |
Spain Neuraxpharm Spain, S.L.U. Tel: +34 93 602 24 21 | Poland Neuraxpharm Polska Sp. z.o.o. Tel: +48 505 499 420 |
France Neuraxpharm France Tel: +33 1.53.62.42.90 | Portugal Neuraxpharm Portugal, Unipessoal Lda Tel: +351 910 259 536 |
Croatia Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 Ireland Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Romania Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 Slovenia Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Iceland Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Slovakia Neuraxpharm Bohemia s.r.o. Tel: +421 255 425 562 |
Italy Neuraxpharm Italy S.p.A. Tel: +39 0736 980619 | Finland Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Cyprus Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | Sweden Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Latvia Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 | United Kingdom (Northern Ireland) Laboratorios Lesvi, S.L. Tel: +34 93 602 24 21 |
Date of last revision of this leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUCCOLAM 2.5 mg ORAL SOLUTIONDosage form: GEL/PASTE/ORAL LIQUID, 10 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 5 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 7.5 mg midazolam hydrochlorideActive substance: midazolamManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription required
Online doctors for BUCCOLAM 2.5 mg ORAL SOLUTION
Discuss questions about BUCCOLAM 2.5 mg ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions