
Midazolamum Aguettant

Ask a doctor about a prescription for Midazolamum Aguettant

How to use Midazolamum Aguettant
Leaflet accompanying the packaging: patient information
Midazolamum Aguettant, 1 mg/mL, solution for injection in a pre-filled syringe
Midazolamum
Read the leaflet carefully before administering the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor, pharmacist, or nurse.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What Midazolamum Aguettant is and what it is used for
- 2. Important information before administering Midazolamum Aguettant
- 3. How Midazolamum Aguettant is administered
- 4. Possible side effects
- 5. How to store Midazolamum Aguettant
- 6. Contents of the packaging and other information
1. What Midazolamum Aguettant is and what it is used for
Midazolamum Aguettant contains midazolam, which belongs to a group of medicines called benzodiazepines. It is a fast-acting medicine used to induce sleep or sedation and to relieve anxiety symptoms and reduce muscle tension. Midazolamum Aguettant is used in adults:
- in general anesthesia to induce or maintain sleep.
Midazolamum Aguettant is also used in adults and children (12 years and older):
- in intensive care units to sedate the patient and induce sleep; this is called "sedation";
- before and during medical procedures or examinations, during which the patient should remain conscious; the medicine makes the patient feel calm and sleepy; this is called "light sedation";
- to calm the patient and make them feel sleepy before receiving an anesthetic.
2. Important information before administering Midazolamum Aguettant
When not to use Midazolamum Aguettant:
Warnings and precautions
The patient's medical history may affect the administration of Midazolamum Aguettant. Before receiving Midazolamum Aguettant, the patient should talk to their doctor or nurse if:
- the patient is over 60 years old,
- the patient has a chronic illness, such as chronic respiratory, kidney, liver, or heart disease,
- the patient has a disease that makes them feel very weak, tired, and lacking energy,
- the patient has muscle weakness (a nerve-muscle disease characterized by muscle weakness),
- the patient has sleep apnea (temporary pauses in breathing during sleep)
- the patient has a history of alcohol abuse
- the patient has a history of drug abuse
If any of these conditions apply to the patient (or if the patient is unsure), they should inform their doctor or nurse before administering Midazolamum Aguettant.
Children
Midazolamum Aguettant can be used in children 12 years and older.
If it is planned to administer this medicine to a child 12 years or older:
- the doctor or nurse should be informed if any of the above points apply to the child (12 years or older).
- in particular, the doctor or nurse should be informed if the child has heart or breathing problems.
Midazolamum Aguettant and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken. This will help the doctor determine the correct dose of Midazolamum Aguettant for the patient.
The following medicines may affect the action of Midazolamum Aguettant:
- antidepressants,
- sleeping pills (that help with falling asleep),
- sedatives (that induce a state of calm and sleepiness),
- calming medicines (used in states of anxiety and to help with falling asleep),
- carbamazepine or phenytoin (used in the treatment of seizures or epilepsy),
- rifampicin (used in the treatment of tuberculosis),
- medicines used to treat HIV infection, known as protease inhibitors (such as saquinavir),
- macrolide antibiotics (such as erythromycin or clarithromycin),
- medicines used to treat fungal infections (ketoconazole, voriconazole, fluconazole, itraconazole, posaconazole),
- strong painkillers,
- atorvastatin (used to treat high cholesterol),
- antihistamines (medicines used to treat allergies),
- St. John's Wort (a herbal medicine used to treat depression),
- medicines used to treat high blood pressure, known as calcium channel blockers (diltiazem). If any of these situations apply to the patient or if they have any doubts, they should inform their doctor or nurse before administering Midazolamum Aguettant.
Midazolamum Aguettant and alcohol
While taking Midazolamum Aguettant, the patient should not drink alcohol, as it may increase sleepiness and cause breathing problems.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before taking this medicine. The doctor will decide whether this medicine is suitable for them.
Women should not breastfeed for 24 hours after receiving Midazolamum Aguettant, as it may pass into breast milk.
Driving and using machines
After receiving Midazolamum Aguettant, the patient should not drive or operate machinery until their doctor decides it is safe to do so.
This medicine may cause sleepiness and memory problems. It may also affect concentration and coordination. These effects may impair the patient's ability to drive or operate machinery.
After receiving this medicine, the patient should always be accompanied by an adult when traveling home.
Midazolamum Aguettant contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per 5 mL pre-filled syringe, which means it is considered "sodium-free".
The medicine contains 33.00 mg of sodium (the main component of common salt) per 10 mL. This corresponds to 1.7% of the maximum recommended daily intake of sodium in the diet for adults.
3. How Midazolamum Aguettant is administered
Midazolamum Aguettant should only be administered by an experienced doctor or nurse. It is administered in a place equipped with appropriate equipment to monitor and treat any adverse reactions. This may be a hospital, clinic, or surgical office, with the possibility of monitoring breathing, heart function, and circulation.
It is not recommended to use Midazolamum Aguettant in infants and children under 12 years of age.
How Midazolamum Aguettant is administered
Midazolamum Aguettant can be administered in the following ways:
- by slow injection into a vein (intravenous injection),
- by injection into a muscle (intramuscular injection).
Dosage
The dose of Midazolamum Aguettant may vary for each patient. The appropriate dose of the medicine for a given patient is determined by the doctor. The dose depends on the patient's age, weight, and overall health. It also depends on the purpose of the treatment and the patient's response to the treatment, as well as whether other medicines are being administered at the same time.
After administering Midazolamum Aguettant
After the procedure, the patient should go home accompanied by an adult, as Midazolamum Aguettant may cause sleepiness, memory problems, decreased concentration, and coordination.
Using a higher dose of Midazolamum Aguettant than recommended
The medicine will be administered by a doctor or nurse, which means there is a low probability of using too high a dose. However, if the patient accidentally overdoses, it may lead to:
- sleepiness and loss of coordination and reflexes,
- speech disorders and involuntary eye movements,
- low blood pressure - this may cause dizziness or fainting,
- slowing or stopping breathing and heart function, and loss of consciousness (coma).
Using Midazolamum Aguettant for long-term sedation in patients in intensive care units
If Midazolamum Aguettant is used for a long time, the following may occur:
- decreased effectiveness of the medicine,
- dependence on the medicine or withdrawal symptoms when treatment is stopped (see "Stopping Midazolamum Aguettant" below).
Stopping Midazolamum Aguettant
If Midazolamum Aguettant has been administered to the patient for a long time, for example, in an intensive care unit, when the treatment is stopped, the patient may experience withdrawal symptoms. These include:
- mood changes
- seizures (convulsions)
- headache
- muscle pain
- sleeping problems (insomnia)
- feeling of anxiety, tension, restlessness, confusion, or bad mood (irritability)
- seeing and possibly hearing things that do not exist (hallucinations). The doctor will gradually reduce the dose. This will help avoid withdrawal symptoms.
4. Possible side effects
Like all medicines, Midazolamum Aguettant can cause side effects, although not everybody gets them.
The following side effects have been reported with an unknown frequency and cannot be estimated from the available data.
If the patient experiences any of the following side effects, they should stop taking Midazolamum Aguettant and immediately inform their doctor. These side effects can be life-threatening and require urgent treatment.
- Severe allergic reaction (anaphylactic shock). Symptoms may include sudden rash, itching, or hives, as well as swelling of the face, lips, tongue, or other parts of the body (angioedema).
- Shortness of breath, wheezing, or difficulty breathing, or pale skin, weak and rapid pulse, or feeling of loss of consciousness. Additionally, there may be chest pain, which can be a sign of a serious allergic reaction called Kounis syndrome.
- Heart attack. The symptom may be chest pain.
- Breathing difficulties, sometimes causing respiratory arrest.
- Spasm of the vocal cords resulting in airway obstruction. Life-threatening side effects are more common in people over 60 years old and in people with breathing or heart problems. These side effects are also more likely if the medicine is injected too quickly or in large doses.
Other side effects
Nervous system disorders and psychiatric disorders
- Decreased alertness.
- Confusion.
- Euphoria (excessive feeling of happiness or excitement).
- Changes in libido.
- Feeling of fatigue, sleepiness, or prolonged sedation.
- Seeing and hearing things that do not exist (hallucinations).
- Headache.
- Dizziness.
- Difficulty coordinating muscle movements.
- Seizures in premature infants and newborns.
- Temporary memory loss. The duration of memory loss depends on the dose used. In individual cases, it may last for a long time.
- Anxiety, hostility, anger, or aggression. Uncontrolled muscle spasms or tremors may also occur. The likelihood of these events is higher if the patient receives a large dose of Midazolamum Aguettant or if the medicine is administered too quickly, as well as in children and the elderly.
The likelihood of these events is higher if the patient receives a large dose of Midazolamum Aguettant or if the medicine is administered too quickly, as well as in children and the elderly.
Cardiovascular disorders
- Fainting.
- Slow heart rate.
- Flushing of the face and neck.
- Low blood pressure. This may cause dizziness or fainting.
Respiratory disorders
- Hiccup.
- Shortness of breath, lack of breath.
Gastrointestinal disorders
- Dry mouth.
- Constipation.
- Nausea, vomiting.
Skin and subcutaneous tissue disorders
- Itching sensation.
- Rash, including hives.
- Redness, pain, thrombophlebitis, or swelling of the skin at the injection site.
General disorders and administration site conditions
- Allergic reactions, including rash and wheezing.
- Withdrawal symptoms (see "Stopping Midazolamum Aguettant" above in section 3).
- Falls and bone fractures. The risk of these events is higher if the patient is also taking other medicines that cause sleepiness (e.g., sedatives or sleeping pills) or if the patient is also drinking alcohol.
Elderly patients
- The risk of falls and bone fractures is higher in elderly patients who receive benzodiazepines, such as Midazolamum Aguettant.
- In patients over 60 years old, there is a higher risk of life-threatening side effects.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Midazolamum Aguettant
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, blister, and label of the pre-filled syringe after "EXP". The expiry date refers to the last day of the month.
Store in the original packaging to protect from light. Do not freeze.
Store the pre-filled syringe in the unopened blister until use.
After opening, the medicinal product should be used immediately.
Do not use this medicine if visible signs of deterioration are observed.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Midazolamum Aguettant contains
- The active substance of the medicine is midazolam. Each mL of the solution for injection contains 1 mg of midazolam. Each 5 mL pre-filled syringe contains 5 mg of midazolam. Each 10 mL pre-filled syringe contains 10 mg of midazolam.
- The other ingredients are sodium chloride, sodium hydroxide, hydrochloric acid, and water for injections.
What Midazolamum Aguettant looks like and contents of the pack
Midazolamum Aguettant is a clear, colorless solution for injection (injection) in a pre-filled syringe (polypropylene) with a capacity of 5 mL or 10 mL with a plunger (chlorobutyl), without a needle, with a self-adhesive, transparent label with a scale (scale every 0.2 mL from 0 to 5 mL or 10 mL). The tip cap (polypropylene) protects the tip of the syringe. Each pre-filled syringe is packaged individually in a blister. The cartons contain 10 pre-filled syringes.
Marketing authorization holder and manufacturer
LABORATOIRE AGUETTANT, 1, rue Alexander Fleming, 69007 Lyon, France.
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Denmark, Finland, France, Germany, Luxembourg, Italy, Netherlands, Norway, Spain, Sweden, Portugal: Midazolam Aguettant
Ireland: Midazolam
Poland: Midazolamum Aguettant
Date of last revision of the leaflet:
------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
The syringe must be carefully prepared as follows:
The pre-filled syringe is intended for use in a single patient only. After use, it should be discarded. It should not be used again.
The contents of the unopened and undamaged blister are sterile, so the blister should not be opened until the syringe is ready for use.
Before administration, the medicinal product should be visually inspected for particles and discoloration. Only a clear, colorless solution free from particles or precipitates should be used.
This medicinal product should not be used if the protective cap on the syringe is damaged.
This medicine should not be used if visible signs of deterioration are observed.
The outer surface of the syringe is sterile until the blister is opened. The blister should not be opened until use.
When handling this medicine with asepsis, after removal from the blister, it may be placed on a sterile surface.
The volume to be administered should be calculated with reference to the appropriate dosing.
- 1) Remove the sterile pre-filled syringe from the blister.
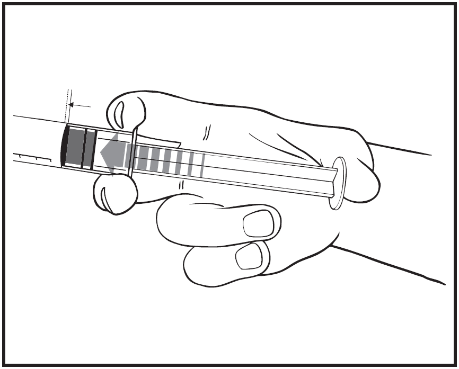
- 2) Press the plunger to release the rubber stopper. The sterilization process may have caused the rubber stopper to stick to the syringe body.
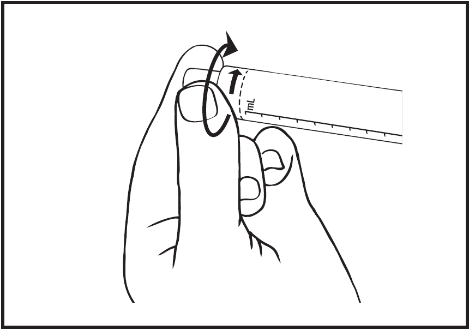
- 3) Unscrew the tip cap to break the seal. The exposed luer connector should not be touched to avoid contamination.
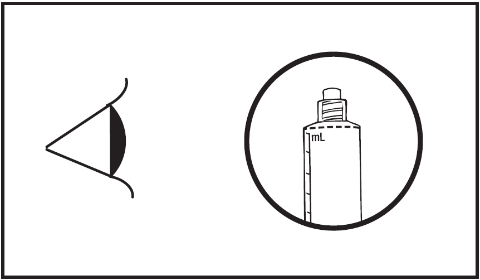
- 4) Check that the tip cap has been completely removed. If not, the protective cap should be put back and rotated again.
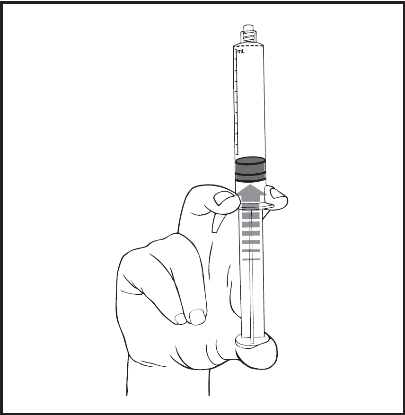
- 5) Release the air by gently pressing the plunger.
- 6) Connect the syringe to the intravenous access using a luer/luer lock system. Slowly press the plunger to inject the required volume. Administer the product according to the recommended route of administration.
The pre-filled syringe contains a medicinal product ready for administration. The pre-filled syringe is not suitable for use with infusion pumps.
The pre-filled syringe should not be used if it has been damaged or if it has been handled without maintaining asepsis.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterLaboratoire Aguettant
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Midazolamum AguettantDosage form: Tablets, 15 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Tablets, 7.5 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Solution, 10 mgActive substance: midazolamManufacturer: MoNo chem-pharm. Produkte GmbHPrescription required
Alternatives to Midazolamum Aguettant in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Midazolamum Aguettant in Spain
Alternative to Midazolamum Aguettant in Ukraine
Online doctors for Midazolamum Aguettant
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Midazolamum Aguettant – subject to medical assessment and local rules.









