
Soloxelam
Ask a doctor about a prescription for Soloxelam

How to use Soloxelam
Leaflet accompanying the packaging: information for the user
Soloxelam, 2.5 mg, oral solution
Soloxelam, 5 mg, oral solution
Soloxelam, 7.5 mg, oral solution
Soloxelam, 10 mg, oral solution
Midazolam
Read the leaflet carefully before administering this medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, tell the doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Soloxelam and what is it used for
- 2. Important information before taking Soloxelam
- 3. How to take Soloxelam
- 4. Possible side effects
- 5. How to store Soloxelam
- 6. Contents of the packaging and other information
1. What is Soloxelam and what is it used for
Soloxelam oral solution contains the active substance midazolam.
Midazolam belongs to a group of medicines known as benzodiazepines.
Soloxelam is used to stop sudden, prolonged seizures in infants from 3 months, children and adolescents, and adults.
In infants from 3 months to less than 6 months, this medicine should only be used in a hospital where the patient's condition can be monitored and resuscitation equipment is available.
This medicine may be administered by parents/caregivers only if the patient has been diagnosed with epilepsy.
2. Important information before taking Soloxelam
When not to take Soloxelam
- if the patient is allergic to midazolam, benzodiazepines (such as diazepam) or any of the other ingredients of this medicine (listed in section 6);
- if the patient has a nerve and muscle disease causing muscle weakness (myasthenia);
- if the patient has significant breathing difficulties at rest (Soloxelam may worsen breathing difficulties);
- if the patient has a disease in which breathing is frequently disturbed during sleep (sleep apnea syndrome);
- if the patient has severe liver function disorders.
Warnings and precautions
Children:
Before starting Soloxelam, discuss with the doctor or pharmacist if the patient has:
- kidney, liver, or heart problems;
- a lung disease that causes constant breathing difficulties.
Adults:
Before starting Soloxelam, discuss with the doctor or pharmacist if:
- the patient is over 60 years old,
- the patient has a chronic disease (e.g., breathing difficulties, kidney, liver, or heart problems),
- the patient is weakened (has a disease that makes them feel very weak, exhausted, and lacking energy).
This medicine may cause patients to forget what happened after taking it. After taking the medicine, patients should be closely monitored.
It is recommended to avoid administering the medicine to patients who abuse alcohol or drugs.
In patients with respiratory or heart function disorders, life-threatening events may occur more frequently, especially after taking high doses of Soloxelam.
Children under 3 months:
Soloxelam should not be given to children under 3 months, as there is not enough information about this age group.
Elderly:
Elderly people are more sensitive to the effects of benzodiazepines.
If there are any doubts about any of the above conditions, consult a doctor or pharmacist before taking this medicine.
Soloxelam with other medicines
Tell the doctor or pharmacist about all medicines the patient is taking, has recently taken, or might take.
It is especially important because taking more than one medicine at the same time may increase or decrease the effects of these medicines.
The effects of Soloxelam may be increased by medicines such as:
- antiepileptic medicines (used to treat epilepsy), such as phenytoin;
- antibiotics, such as erythromycin, clarithromycin;
- antifungal medicines, such as ketoconazole, voriconazole, fluconazole, itraconazole, posaconazole;
- medicines used to treat stomach ulcers, such as cimetidine, ranitidine, and omeprazole;
- medicines used to treat high blood pressure, such as diltiazem, verapamil;
- certain medicines used to treat HIV and AIDS, such as saquinavir, the combination of lopinavir and ritonavir;
- opioid painkillers (very strong painkillers), such as fentanyl;
- medicines used to lower blood fat levels, such as atorvastatin;
- medicines used to treat nausea, such as nabilone;
- sleeping pills;
- antidepressants with a sedative effect (medicines used to treat depression that cause drowsiness);
- sedatives;
- anesthetics (used to relieve pain);
- antihistamines (used to treat allergies).
The effects of Soloxelam may be decreased by medicines such as:
- rifampicin (used to treat tuberculosis);
- xanthines (used to treat asthma);
- St. John's Wort (a herbal medicine). You should avoid taking this medicine while taking Soloxelam.
Soloxelam may increase the effects of certain muscle relaxants, such as baclofen (causing increased drowsiness). This medicine may also inhibit the effects of certain medicines, such as levodopa (a medicine used to treat Parkinson's disease).
Consult a doctor or pharmacist for information on which medicines to avoid while taking Soloxelam.
Soloxelam with food and drink
Do not drink alcohol while taking Soloxelam. Alcohol may increase the sedative effect of the medicine and cause increased drowsiness.
Do not drink grapefruit juice while taking Soloxelam. Grapefruit juice may increase the sedative effect of the medicine and cause increased drowsiness.
Pregnancy
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult a doctor before taking this medicine.
Taking high doses of Soloxelam in the last 3 months of pregnancy may cause heart rhythm disorders in the unborn baby. Babies born to mothers who took this medicine during childbirth may be born with a weak sucking reflex, breathing difficulties, and decreased muscle tone.
Breastfeeding
Tell the doctor if the patient is breastfeeding. Small amounts of Soloxelam may pass into breast milk, but it may not be necessary to stop breastfeeding. The doctor will decide whether the patient can breastfeed after taking this medicine.
Driving and using machines
Soloxelam may cause drowsiness, forgetfulness, or decreased concentration and coordination. This may affect the performance of learned tasks, such as driving, cycling, or operating machinery.
After taking this medicine, do not drive, cycle, or operate machinery until fully recovered. Consult a doctor if further information is needed.
Soloxelam contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per oral syringe, which means it is essentially 'sodium-free'.
3. How to take Soloxelam
Always take this medicine exactly as the doctor has told you. If you are not sure, consult a doctor or pharmacist.
Dosage
The doctor will prescribe the correct dose of Soloxelam, usually based on the patient's age. Different doses are marked with different colors on the box, tube, and oral syringe.
Depending on the age, the patient will receive one of the following doses from a color-coded packaging:
| Age range | Dose | Label color |
| from 3 months to less than 1 year | 2.5 mg | Yellow |
| from 1 year to less than 5 years | 5 mg | Blue |
| from 5 years to less than 10 years | 7.5 mg | Purple |
| from 10 years and for adults | 10 mg | Orange |
The dose is the entire contents of one oral syringe. Do not take more than one dose.
Infants from 3 months to less than 6 months should only be treated in a hospital where the patient's condition can be monitored and resuscitation equipment is available.
Preparing the medicine for administration
If the patient has a seizure, allow free movement of the body and do not try to restrain it. Only move the patient if they are in danger, e.g., near deep water, fire, or sharp objects.
Secure the patient's head with something soft, such as a pillow or by placing the patient's head on your knees.
Check that the dose of the medicine is correct for the patient and corresponds to the patient's age.
How to administer the medicine
Consult a doctor, pharmacist, or nurse to show you how to take or administer the medicine. If you have any doubts, always consult them for advice.
Information on how to administer the medicine is also on the tube label.
Do not inject Soloxelam. Do not put a needle on the oral syringe.
Step 1
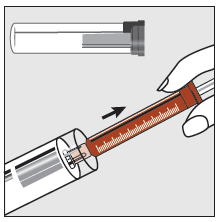
Holding the plastic tube, break the seal at one end and remove the cap. Remove the syringe from the tube.
Step 2
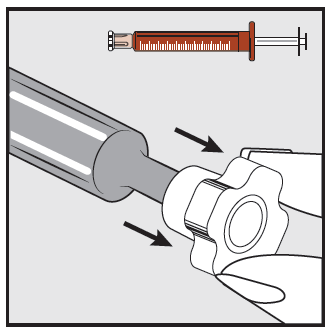
Remove the transparent cap from the end of the syringe and dispose of it safely.
Step 3
Gently grasp the patient's cheek with your thumb and finger, and pull it back. Insert the tip of the syringe deeply between the inside of the cheek and the lower gum.
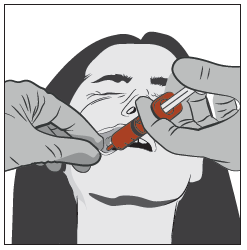
Step 4
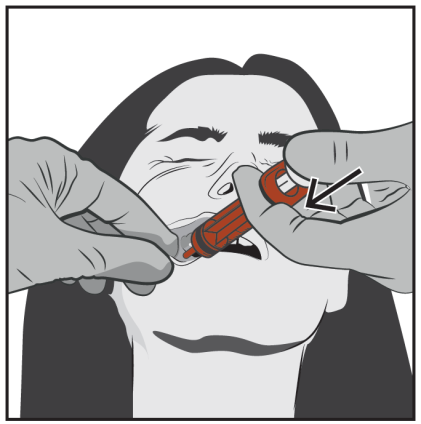
Slowly press the plunger until it stops.
The entire volume of the solution should be slowly administered into the space between the gum and cheek (into the mouth).
If necessary (in case of larger volumes and/or smaller patients), slowly administer about half of the dose on one side of the mouth and then slowly administer the remaining half on the other side of the patient's mouth.
When to call emergency services
Always follow the doctor's or medical staff's instructions. If you have any doubts, immediately call for medical help if:
- the seizure does not stop within 10 minutes;
- it is impossible to empty the syringe or some of its contents spill;
- the patient starts breathing more slowly or stops breathing, i.e., breathing becomes slow and shallow or the patient's lips turn blue;
- there are symptoms of a heart attack, which may include chest pain that may radiate to the neck, shoulders, and down to the left arm;
- the patient vomits and the seizure does not stop within 10 minutes;
- too much Soloxelam has been administered and there are symptoms of overdose, including: drowsiness, fatigue, feeling of exhaustion; confusion or disorientation; lack of knee-jerk reflex or lack of reaction to pinching; breathing difficulties (slow or shallow breathing); low blood pressure (dizziness and feeling of fainting); coma.
Keep the syringe to show it to the emergency services or doctor.
Do not administer a dose of Soloxelam larger than prescribed by the doctor.
If the patient vomits
- Do not administer another dose of Soloxelam to the patient.
- If the seizure does not stop within 10 minutes, call emergency services.
If you have any further doubts about taking this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Soloxelam can cause side effects, although not everybody gets them.
Serious side effects
Immediately contact a doctor or call emergency services if the patient experiences:
- serious breathing difficulties, such as slow and shallow breathing or blue lips. In very rare cases, breathing may stop;
- a heart attack. Symptoms may include chest pain that may radiate to the neck, shoulders, and down to the left arm;
- swelling of the face, lips, tongue, or throat, which makes swallowing or breathing difficult, or paleness of the skin, weak and rapid pulse, or feeling of fainting. This may be a serious allergic reaction.
Other side effects
If side effects occur, consult a doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet.
Common side effects (may occur in less than 1 in 10 people):
- nausea and vomiting;
- drowsiness or loss of consciousness.
Uncommon side effects (may occur in less than 1 in 100 people):
- rash, hives (urticaria), itching.
Rare side effects (may occur in less than 1 in 10,000 people):
- agitation, restlessness, hostility, anger, or aggression, excitement, confusion, euphoria (excessive feeling of happiness or excitement) or hallucinations (seeing and hearing things that do not exist in reality);
- muscle spasms and muscle tremors (involuntary muscle contractions);
- decreased alertness;
- headache;
- dizziness;
- coordination difficulties;
- seizures (convulsions);
- temporary memory loss. The duration depends on the amount of Soloxelam administered to the patient;
- low blood pressure, slow heart rate, or flushing of the face and neck (hot flashes);
- laryngospasm (constriction of the vocal cords causing breathing difficulties and loud breathing);
- constipation;
- dry mouth;
- fatigue;
- hiccups.
Reporting side effects
If side effects occur, including any not listed in this leaflet, tell the doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Soloxelam
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, tube, and oral syringe after EXP. The expiry date refers to the last day of that month.
Store the oral syringe in its protective plastic tube.
Do not store above 30°C.
Do not use this medicine if the packaging is already open or damaged.
Disposal of oral syringes
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Soloxelam contains
- The active substance is midazolam.
- 2.5 mg - each oral syringe contains 2.5 mg of midazolam (as hydrochloride) in 0.5 mL of solution.
- 5 mg - each oral syringe contains 5 mg of midazolam (as hydrochloride) in 1 mL of solution.
- 7.5 mg - each oral syringe contains 7.5 mg of midazolam (as hydrochloride) in 1.5 mL of solution.
- 10 mg - each oral syringe contains 10 mg of midazolam (as hydrochloride) in 2 mL of solution.
Other ingredients are: sodium chloride, purified water, hydrochloric acid, and sodium hydroxide (to adjust pH).
What Soloxelam looks like and contents of the pack
2.5 mg - packaging marked with yellow color.
5 mg - packaging marked with blue color.
7.5 mg - packaging marked with purple color.
10 mg - packaging marked with orange color.
Soloxelam is a clear oral solution.
The medicine is supplied in an orange prefilled oral syringe without a needle, with a plunger and cap.
Each oral syringe is individually packaged in a protective plastic tube.
Soloxelam is available in cardboard boxes containing 2 or 4 prefilled oral syringes (each containing the same dose).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Exeltis Poland Sp. z o.o.
Szamocka 8
01-748 Warsaw
e-mail: [email protected]
Manufacturer
Laboratorios Liconsa S.A.
Avda. Miralcampo, 7
Poligono Industrial Miralcampo
19200 Azuqueca de Henares
Guadalajara
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Sweden:
Midazolam Medical Valley 2.5 mg, 5 mg, 7.5 mg, 10 mg oral solution.
Finland:
Midazolam Medical Valley 2.5 mg, 5 mg, 7.5 mg, 10 mg solution for oral use.
Germany:
Midazolam Desitin 2.5 mg, 5 mg, 7.5 mg, 10 mg solution for oral use.
Norway:
Midazolam Medical Valley 2.5 mg, 5 mg, 7.5 mg, 10 mg oral solution.
Netherlands:
Midazolam Xiromed 2.5 mg, 5 mg, 7.5 mg, 10 mg solution for oromucosal use.
Denmark:
Midazolam Medical Valley 2.5 mg, 5 mg, 7.5 mg, 10 mg oral solution.
Iceland:
Midazolam Medical Valley 2.5 mg, 5 mg, 7.5 mg, 10 mg oral solution.
France:
Midazolam Liconsa 2.5 mg, 5 mg, 7.5 mg, 10 mg buccal solution.
Ireland:
Midazolam Rowa 2.5 mg, 5 mg, 7.5 mg, 10 mg oromucosal solution.
Romania:
Midazolam Desitin 2.5 mg, 5 mg, 7.5 mg, 10 mg buccal solution.
Spain:
Oroxelam 2.5 mg, 5 mg, 7.5 mg, 10 mg oromucosal solution.
Poland:
Soloxelam.
Italy:
Oroxelam.
Date of last revision of the leaflet:13.03.2025
Other sources of information
Detailed information on this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: http://www.urpl.gov.pl.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratorios Liconsa, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SoloxelamDosage form: Tablets, 15 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Tablets, 7.5 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Solution, 10 mgActive substance: midazolamManufacturer: MoNo chem-pharm. Produkte GmbHPrescription required
Alternatives to Soloxelam in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Soloxelam in España
Alternative to Soloxelam in Ucrania
Online doctors for Soloxelam
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Soloxelam – subject to medical assessment and local rules.









