
OCTANATE LV 100 IU/ML POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use OCTANATE LV 100 IU/ML POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Octanate LV 100 UI/ml, powder and solvent for solution for injection
Human coagulation factor VIII
Octanate LV 200 UI/ml, powder and solvent for solution for injection
Human coagulation factor VIII
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Octanate LV and what is it used for
- What you need to know before you use Octanate LV
- How to use Octanate LV
- Possible side effects
- Storage of Octanate LV
- Contents of the pack and further information
1. What is Octanate LV and what is it used for
Octanate LV belongs to a group of medicines called coagulation factors and contains human coagulation factor VIII. This is a special protein involved in blood clotting.
Octanate LV is used to treat and prevent bleeding in patients with hemophilia A. This is a disease where bleeding can last longer than expected. It is due to a hereditary lack of factor VIII in the blood.
2. What you need to know before you use Octanate LV
It is highly recommended that each time you receive a dose of Octanate LV, you note the name and batch number of the product in order to maintain a record of the batches used.
Your doctor may recommend that you consider vaccination (against hepatitis A and B) if you regularly or repeatedly receive human-derived factor VIII products.
Do not use Octanate LV:
if you are allergic to human coagulation factor VIII or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Octanate LV.
Octanate LV contains very small amounts of other human proteins. Any medicine that contains proteins and is injected into a vein (administered intravenously) can cause allergic reactions (see section 4, "Possible side effects").
The formation of inhibitors (antibodies) is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially in large quantities, can prevent the treatment from working properly, so you and your child will be carefully monitored for the development of such inhibitors. If your bleeding or your child's bleeding is not being controlled with Octanate LV, contact your doctor immediately.
Information about the blood and plasma used for Octanate LV
When medicines derived from human plasma or blood are administered, certain measures must be taken to avoid the transmission of infectious diseases to patients. Such measures include careful selection of donors to exclude those at risk of being carriers of infectious diseases, testing for specific infection markers in individual donations and plasma pools, as well as the inclusion of stages in the manufacturing process to eliminate/inactivate viruses. Despite this, when medicines derived from human blood or plasma are administered, the possibility of transmitting infectious agents cannot be completely excluded. This also applies to emerging or unknown viruses or other types of infections.
These measures are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV). The measures taken may have limited value against non-enveloped viruses, such as parvovirus B19.
Parvovirus B19 infection can be severe for a pregnant woman (fetal infection) and for individuals whose immune system is depressed or for patients with certain types of anemia (e.g., sickle cell disease or abnormal red blood cell destruction).
Using Octanate LV with other medicines:
Tell your doctor or pharmacist if you are using or have recently used or might use other medicines, including those obtained without a prescription.
No interactions of human coagulation factor VIII with other medicines are known. However, Octanate LV should not be mixed with other medicines during infusion.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines:
No effects on the ability to drive or use machines have been observed.
Octanate LV contains
up to 40 mg of sodium (main component of table/cooking salt) per vial. This is equivalent to 2% of the maximum recommended daily sodium intake for an adult.
3. How to use Octanate LV
Octanate LV should be administered intravenously once reconstituted with the provided solvent.
Treatment should be initiated under medical supervision.
Dose to prevent bleeding.If you have severe hemophilia A, you should be injected with 20 to 40 IU of factor VIII per kilogram of body weight every two or three days for long-term prevention. Your dose will be adjusted based on your response. In some cases, shorter administration intervals or higher doses may be necessary.
Dose calculation:
Follow your doctor's instructions for administering Octanate LV exactly. Consult your doctor or pharmacist if you have any doubts.
Factor VIII activity refers to the amount of factor VIII present in the plasma. It is expressed as a percentage (relative to normal human plasma) or in International Units (IU). The dose of factor VIII is expressed in IU.
One International Unit (IU) of factor VIII activity is equivalent to the amount of factor VIII in one milliliter of normal human plasma. One IU of factor VIII per kilogram of body weight increases the plasma factor VIII activity by 1.5-2% of normal activity. To calculate the dose you need, you must determine the level of factor VIII activity in your plasma. This will indicate the amount of activity that needs to be increased. Ask your doctor if you have any doubts about the amount of factor VIII activity you need to increase or how to calculate your dose.
The required dosage is determined using the following formula:

Units needed = body weight (kg) x desired factor VIII increase (%) (IU/dl) x 0.5
The amount to be administered and the frequency of administration should always be guided by clinical efficacy for each individual patient.
In the case of the following bleeding episodes, the factor VIII activity should not fall below the given plasma activity level (in % of normal) within the corresponding period.
The following table can be used as a dosing guide for bleeding episodes and surgery:
Severity of bleeding /Type of surgical procedure | Required factor VIII level(%) (IU/dl) | Dose frequency (hours between doses) /Duration of therapy (in days) |
Bleeding: | ||
Joint bleeding (early hemarthrosis), muscle bleeding, or oral bleeding. | 20 - 40 | Repeat every 12 to 24 hours. At least 1 day, until pain decreases or healing is achieved. |
More extensive joint bleeding (hemarthrosis), muscle bleeding, or blood effusion (hematoma). | 30 - 60 | Repeat infusion every 12 to 24 hours for 3-4 days or more until pain and disability are resolved. |
Life-threatening bleeding, such as cerebral surgery, throat bleeding, severe abdominal bleeding. | 60 - 100 | Repeat infusion every 8 to 24 hours until the danger has passed. |
Surgery: | ||
Minorincluding dental extraction. | 30 - 60 | Every 24 hours, at least 1 day, until healing is achieved. |
Major | 80 – 100 (before and after an operation) | Repeat infusion every 8-24 hours until adequate wound healing, followed by therapy for at least 7 days to maintain a factor VIII activity of 30% to 60%. |
Your doctor will indicate the dose and frequency at which you should use Octanate LV.
Your response to factor VIII products may vary. Therefore, factor VIII levels should be determined during treatment to calculate the correct dose and adequate infusion frequency.
Use in children
Clinical studies did not identify any special dosing requirements for children. For both treatment and prophylaxis, the dosing is the same for adults and children.
Instructions for outpatient treatment
- Please read all the instructions and follow them carefully.
- Do not use Octanate LV after the expiration date stated on the packaging.
- During the procedure described below, sterility must be maintained.
- Visually inspect the reconstituted medicine to check for particles or color change before administration.
- The solution should be clear or slightly opalescent. Do not use cloudy solutions or those containing sediment.
- Use the prepared solution immediately to avoid microbial contamination.
- Use only the infusion equipment provided. Using other injection/infusion equipment may cause additional risk and treatment failure.
Instructions forpreparingthesolution:
1.
- Remove the flip-off caps from the vials and clean the rubber stoppers with one of the alcohol swabs provided.
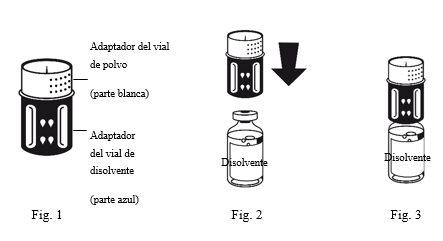
- The transfer device is shown in Fig. 1. Place the solvent vial on a flat surface and hold it firmly. Take the transfer device and turn it upside down. Place the blue part of the transfer device over the top of the solvent vial and press firmly until you hear a click (Fig. 2 + 3). Do not twist when attaching.
|
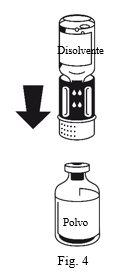
The dissolution is complete in less than 10 minutes at room temperature. A slight foam may appear during preparation. Unscrew the two parts of the transfer device (Fig. 5). The foam will disappear. Discard the empty solvent vial along with the blue part of the transfer device. |
Instructions for injection:
As a precaution, your pulse rate should be measured before and during the injection. If your pulse rate increases significantly, reduce the injection rate or interrupt administration for a brief period.
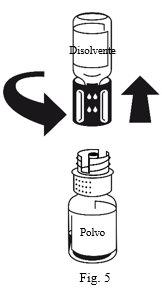
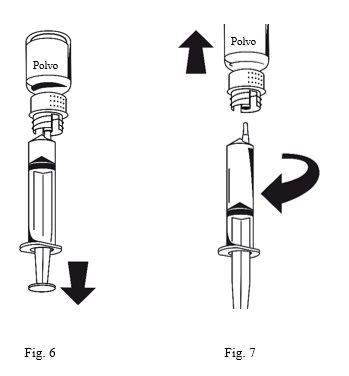
- Attach the syringe to the white part of the transfer device. Turn the vial upside down and withdraw the solution into the syringe (Fig.6). The solution should be clear or slightly opalescent. Once the solution has been transferred, hold the syringe plunger firmly (keeping it down) and withdraw the syringe from the transfer device (Fig. 7). Discard the empty vial along with the white part of the transfer device.
- Clean the injection site with one of the alcohol swabs provided.
- Attach the provided infusion equipment to the syringe.
.
- Insert the injection needle into the chosen vein. If you have used a tourniquet to make the vein more visible, this tourniquet should be released before starting the injection of Octanate LV.
- No blood should flow into the syringe due to the risk of fibrin clot formation.
- Inject the solution into the vein at a slow rate, not exceeding 2-3 ml per minute.
If you use more than one vial of Octanate LV powder for a treatment, you can use the same injection kit and the same syringe. The transfer device is for single use.
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
If you use more Octanate LV than you should
No symptoms of overdose with human coagulation factor VIII have been reported. However, it is recommended not to exceed the recommended dose.
If you forget to use Octanate LV
Do not take a double dose to make up for a forgotten dose. Proceed to administer the next dose immediately and continue with your doctor's or pharmacist's recommendations.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Octanate LV can cause side effects, although not everybody gets them.
Although rare(affects 1 to 10 patients out of 10,000), hypersensitivity or allergic reactions have been observed in patients treated with products containing factor VIII.
Contact your doctor if you experience any of the following symptoms:
discomfort (vomiting), irritation, and itching at the injection site, chest tightness, chills, rapid heartbeat (tachycardia), nausea, tingling sensation (tingling), flushing, headache, hives, low blood pressure (hypotension), skin rash, restlessness, swelling of the face, lips, mouth, tongue, or throat that may cause difficulty swallowing or breathing (angioedema), fatigue (lethargy), wheezing.
In very rarecases (affects less than 1 patient out of 10,000), this hypersensitivity can lead to a life-threatening allergic reaction called anaphylaxis, which can include shock, in addition to some or all of the symptoms described above. In this case, contact your doctor immediately or call an ambulance.
Other rare side effects (affects 1 to 10 patients out of 10,000)
Fever
In children who have not received previous treatment with factor VIII medicines, inhibitor antibodies (see section 2) may occur very frequently (more than 1 in 10 patients); however, in patients who have received previous treatment with factor VIII (more than 150 days of treatment), the risk is infrequent (less than 1 in 100 patients). If this happens, the medicines you or your child are taking may stop working properly, and you or your child may experience persistent bleeding. In this case, contact your doctor immediately.
For information regarding viral safety, see section 2 (Take special care with Octanate LV – information about the blood and plasma used for Octanate LV).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet.
You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Octanate LV
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
Store in a refrigerator (between +2 - 8°C).
Do not freeze.
Keep the vials in the outer packaging to protect them from light.
Use Octanate LV immediately after reconstitution and in a single occasion.
Do not use this medicine if you observe cloudy solutions or those that are not completely dissolved.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Octanate LV
The active ingredient is human coagulation factor VIII.
Volume and concentrations:
Octanate LV powder vial quantity (FVIII IU) | Diluent vial quantity (to be added to the Octanate LV powder vial) (ml) | Nominal concentration of the reconstituted solution (FVIII IU/ml) |
500 IU | 5 | 100 |
1000 IU | 5 | 200 |
The other componentsare:
For the powder: sodium citrate, sodium chloride, calcium chloride, and glycine.
For the solvent: water for injectable preparations.
Appearance of the Product and Container Contents
Octanate LV is presented as a powder and solvent for injectable solution.
The powder is white or pale yellow, or also has the appearance of a friable mass.
The solvent is a clear and colorless liquid.
The available container sizes differ in the amount of human coagulation factor VIII and solvent:
100 IU/ml powder and solvent:
- Powder, 500 IU, in a vial, with a stopper and a flip-off cap.
- Solvent, 5 ml in a vial, with a stopper and a flip-off cap.
- 1 pack of equipment for intravenous injection (1 transfer device, 1 infusion device, 1 disposable syringe)
- 2 alcohol swabs.
200 IU/ml powder and solvent:
- Powder, 1000 IU, in a vial, with a stopper and a flip-off cap.
- Solvent, 5 ml in a vial, with a stopper and a flip-off cap.
- 1 pack of equipment for intravenous injection (1 transfer device, 1 infusion device, 1 disposable syringe)
- 2 alcohol swabs.
Not all container sizes may be available.
Marketing Authorization Holder
Octapharma S.A.
Avda. Castilla, 2. (P.E. San Fernando)
Ed. Dublin, 2ª Planta
28830 San Fernando de Henares
Madrid
Manufacturer:
Octapharma Pharmazeutika Produktionsges.m.b.H.Oberlaaer Str. 235A-1100 ViennaAustria
or
Octapharma S.A.S70 - 72 Rue du Maréchal FochBP 33, F - 67381 LingolsheimFrance
or
Octapharma ABLars Forssells gata 23, 112 75 StockholmSweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
- Octanate LV: Austria, Cyprus, Denmark, France, Netherlands, Malta, Poland, Romania, Spain, Sweden, United Kingdom.
- Octafil LV: Finland
- Octanate: Belgium, Czech Republic, Germany, Ireland, Italy, Latvia, Lithuania, Luxembourg, Portugal
- Octanate Kons: Republic of Slovenia
Date of the Last Revision of this Leaflet: December 2022
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)
http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OCTANATE LV 100 IU/ML POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for OCTANATE LV 100 IU/ML POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about OCTANATE LV 100 IU/ML POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















