
Beriate 500

Ask a doctor about a prescription for Beriate 500

How to use Beriate 500
LEAFLET INCLUDED IN THE PACKAGING: INFORMATION FOR
THE USER
Beriate
Beriate 250, 250 IU Powder and solvent for solution for injection/infusion Beriate 500, 500 IU Powder and solvent for solution for injection/infusion Beriate 1000, 1000 IU Powder and solvent for solution for injection/infusion Beriate 2000, 2000 IU Powder and solvent for solution for injection/infusion Human coagulation factor VIII
You should carefully read the contents of this leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Beriate and what is it used for
- 2. Important information before using Beriate
- 3. How to use Beriate
- 4. Possible side effects
- 5. How to store Beriate
- 6. Contents of the pack and other information
1. What is Beriate and what is it used for
What is Beriate?
Beriate consists of powder and solvent. The reconstituted solution is for intravenous injection or infusion.
Beriate is made from human plasma (the liquid part of the blood) and contains human coagulation factor VIII. It is used to prevent and treat bleeding caused by a lack of factor VIII (haemophilia type A) in the blood. It can also be used to treat acquired factor VIII deficiency.
What is Beriate used for?
Factor VIII is involved in the blood clotting process. A lack of factor VIII means that the blood does not clot as quickly as it should, resulting in an increased tendency to bleed. Replacing factor VIII with Beriate temporarily improves the blood clotting process.
2. Important information before using Beriate
The following sections contain information that you should consider before using Beriate.
When not to use Beriate
- If the patient is allergic (hypersensitive) to the active substance (human coagulation factor VIII) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Identifiability
It is strongly recommended that each time Beriate is administered to the patient, the date of administration, batch number, and volume injected should be recorded in the patient's treatment diary.
Before starting treatment with Beriate, this should be discussed with the doctor or pharmacist.
- It is possible that allergic reactions may occur. Patients should be informed by the doctor about early signs of allergic reactions, such as hives, generalised rash, tightness of the chest, wheezing, low blood pressure, and anaphylaxis (a severe allergic reaction that can cause serious breathing difficulties or dizziness). If such symptoms occur, patients should immediately stop using the product and contact their doctor.
- The formation of inhibitors(antibodies) is a known complication that can occur during treatment with all factor VIII products. These inhibitors, especially at high levels, can disrupt the normal treatment and the patient will be closely monitored for the development of these inhibitors. If bleeding in the patient is not properly controlled with Beriate, the doctor should be informed immediately.
- In the case of existing heart disease or risk of its occurrence, the doctor or pharmacist should be informed.
- If the administration of Beriate requires a central venous access device (CVAD), the doctor should consider the risk of complications associated with CVAD, including local infections, the appearance of bacteria in the blood (bacteraemia), and the formation of blood clots in the blood vessels (thrombosis) at the site of catheter insertion.
The doctor should carefully weigh the benefits of treatment with Beriate against the risk of these complications.
Safety of use in terms of the possibility of transmitting viruses
In the case of medicines produced from human blood or plasma, appropriate preventive measures are taken to prevent the transmission of infectious diseases to patients.
These measures include careful selection of blood and plasma donors to exclude carriers of infectious diseases, as well as testing each donation and plasma pool for viruses/infections.
Manufacturers of these products also include procedures in the production process that inactivate or remove viruses or other pathogens. Despite the use of these measures, when administering medicines derived from human blood or plasma, it is not possible to completely exclude the possibility of transmitting infectious agents. This also applies to unknown or newly discovered viruses and other types of infections.
The safety measures used are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV, the virus that causes AIDS), hepatitis B and C viruses, as well as against non-enveloped viruses, such as hepatitis A virus and parvovirus B19.
In the case of regular or repeated administration of products derived from human plasma (e.g. factor VIII), the doctor may recommend vaccination against hepatitis A and B.
Beriate and other medicines
- The doctor or pharmacist should be informed about all medicines that the patient is currently taking or has recently taken, as well as any medicines that the patient plans to take.
- Beriate should not be mixed with other medicinal products, diluents, and solvents, except those recommended by the manufacturer (see section 6).
Pregnancy, breastfeeding, and fertility
- If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
- Beriate should be used during pregnancy and breastfeeding only if clearly necessary.
- Data on fertility are not available.
Driving and using machines
Beriate does not affect the ability to drive and use machines.
Beriate contains sodium
Beriate 250 IU and 500 IU contain less than 1 mmol of sodium (23 mg) per vial, so it can be considered "essentially sodium-free".
Beriate 1000 IU and 2000 IU contain 27.55 mg of sodium (the main component of common salt) per vial. This is equivalent to 1.4% of the recommended maximum daily intake of sodium for an adult.
3. How to use Beriate
This medicine should always be used as directed by the doctor or pharmacist. If you are unsure, you should consult your doctor or pharmacist.
Treatment of haemophilia A should be started and supervised by a doctor experienced in the treatment of this type of disorder.
Recommended dose
The required amount of factor VIII and the duration of treatment depend on several factors, such as body weight, disease severity, location, and intensity of bleeding or the need to prevent bleeding during surgery or examination.
If the doctor has recommended home use of Beriate, the doctor should inform the patient about how to inject the medicine and what dose to use.
Instructions received from the doctor or nurse at the haemophilia treatment centre must be followed
carefully.
Use in children and adolescents
The dose is determined based on body weight, using the same principle as for adults.
Overdose of Beriate
No symptoms of factor VIII overdose have been reported.
Missed dose of Beriate
The next dose should be administered immediately and continued at regular intervals as directed by the doctor. A double dose should not be administered to make up for a missed dose.
Reconstitution and administration method General recommendations:
- The powder should be mixed (reconstituted) with the solvent (the liquid part) and drawn up from the vial under aseptic conditions.
- The reconstituted solution should be clear or slightly opalescent, i.e. it may shimmer when observed in light. Sometimes, single strands or particles may appear in the vial. The filter, which is part of the Mix2Vial device, removes solid particles. Filtration does not affect the dose calculation. Before administration, after filtration and drawing up into a syringe (see below), the solution should be visually inspected for the presence of small particles and discoloration. Do not use the solution if it is cloudy or contains sediment or particles in the syringe.
- After transfer to a syringe, the product should be used immediately. The product should not be stored in a syringe.
- Any unused product or waste material should be disposed of in accordance with local regulations and the doctor's instructions.
Reconstitution:
Without opening the vials, warm the Beriate powder and solvent to room temperature or body temperature. This can be achieved by leaving the vials at room temperature for about an hour or by holding them in your hands for a few minutes.
Do not expose the vials to direct heat sources.The vial should not be heated to a temperature above body temperature (37°C).
Carefully remove the protective caps from the vials of powder and solvent, wipe the rubber stoppers with an alcohol swab, and let them dry before opening the Mix2Vial package, then follow the instructions below.
- 1. Open the Mix2Vial package by removing the cap. Do notremove the Mix2Vial from the blister!
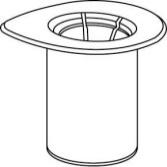
- 2. Place the vial of solventon a flat and clean surface and hold it firmly. Do not remove the Mix2Vial from the blister. Pressing the tip of the blue connector vertically downwardspierce the stopper of the solvent vial.
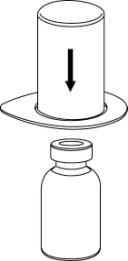
- 3. Carefully remove the blister from the Mix2Vial by holding the edge and pulling verticallyupwards. Make sure to remove only the blister, not the entire Mix2Vial.
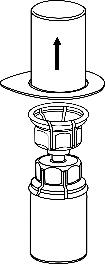
4 .Place the vial of product on a flat and hard surface.
Turn the vial of solvent with the attached Mix2Vial upside down and, pressing the tip of the transparent connector vertically downwards, pierce the stopper of the vial of product. The solvent will flow into the vial of product.
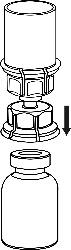
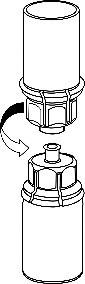
- 5. Holding the vial of medicinal product attached to the Mix2Vial with one hand and the vial of solvent also attached to the Mix2Vial with the other hand, carefully unscrew the Mix2Vial into two parts by twisting in the opposite direction to the arrow. Remove the vial of solvent with the attached blue connector of the Mix2Vial.
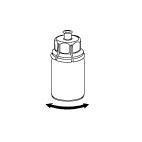
- 6. Bring the powder to complete dissolution by gently rotating the vial of product with the attached transparent connector. Do not shake.
7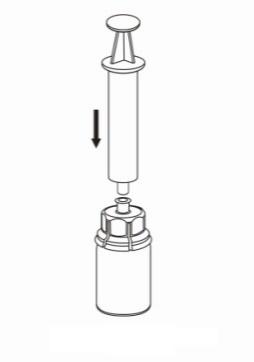 |
|
Withdrawal and administration:
- 8. Holding the plunger of the syringe in, turn the entire set upside down and fill the syringe with the solution, slowly pulling back the plunger of the syringe.
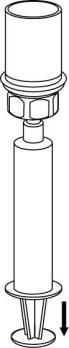
- 9. The solution is now in the syringe. Holding the syringe cylinder (with the plunger facing downwards) firmly, detach the transparent connector of the Mix2Vial from the syringe by twisting in the opposite direction to the arrow.
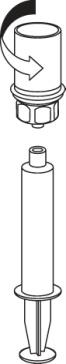
Insert the needle into a vein using the provided venipuncture set. Allow blood to flow into the end of the tube. Attach the syringe to the threaded, securing end of the venipuncture set. According to the doctor's instructions, slowly injectthe reconstituted solution intravenously. The injection or infusion rate should not exceed 2 ml per minute. Care should be taken to ensure that blood does not enter the syringe containing the product.
If it is necessary to administer a larger volume, this can also be done by infusion. To do this, the product should be transferred to an approved infusion set after reconstitution. The infusion should be performed according to the doctor's instructions.
Attention should be paid to the occurrence of any side effects. If such an effect may be related to the administration of Beriate, the injection or infusion should be discontinued (see also section 2).
In case of any further doubts about the use of this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following symptoms occur, the doctor or emergency services or the haemophilia treatment centre at the nearest hospital should be notified immediately:
- Symptoms of angioedema, such as
- swelling of the face, tongue, or throat
- difficulty swallowing
- hives and difficulty breathing The above side effects are very rare (may occur in less than 1 in 10,000 patients) and in some cases may lead to severe allergic reactions (anaphylaxis), including shock.
- Loss of efficacy of treatment (persistent bleeding). In the case of previously untreated children with factor VIII products, inhibitory antibodies (see section 2) may occur very frequently (more than 1 in 10 patients). However, in patients who have previously been treated with factor VIII (more than 150 days of treatment), the risk is not very common (less than 1 in 100 patients). If this happens, the patient's medicines may stop working properly and the patient may experience persistent bleeding. If this happens, the doctor should be notified immediately.
Other side effects:
- Allergic reactions (hypersensitivity), which may include:
- burning and stinging at the injection or infusion site
- chills, fever, generalised rash, blisters
- headache
- low blood pressure, anxiety, rapid heartbeat, tightness in the chest, wheezing
- feeling of fatigue (lethargy)
- nausea, vomiting
- tingling The above side effects are very rare and in some cases may lead to severe allergic reactions (anaphylaxis), including shock.
- Very rarely, elevated body temperature has been observed.
Side effects in children and adolescents
The frequency, type, and severity of adverse reactions in children are comparable to those in adults.
Reporting of side effects
If side effects occur, including any not listed in this leaflet, the doctor or pharmacist or nurse should be informed.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorisation holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Beriate
Do not use this medicine after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of that month.
The batch number is stated on the label and carton after Lot.
- Store in a refrigerator (2°C - 8°C).
- During the shelf life, Beriate may be stored at a temperature not exceeding 25°C for a total period of 1 month. Individual periods of storage at room temperature should be recorded in the treatment records so that the total storage period at this temperature does not exceed one month.
- Beriate does not contain a preservative and should be administered immediately after reconstitution.
- If the reconstituted product is not administered immediately, its storage time in the vial should not exceed 8 hours at room temperature. After transfer to a syringe, the product should be used immediately.
- Do not freeze.
- Store the container in the outer packaging to protect from light.
- The medicine should be stored out of the sight and reach of children.
6. Contents of the pack and other information
What Beriate contains The active substance of Beriate is:
Beriate is available in powder form (containing nominally 250 IU, 500 IU, 1000 IU, or 2000 IU of human coagulation factor VIII per vial) and liquid part (solvent). The reconstituted solution is for injection or infusion.
Beriate 250/500/1000 reconstituted with 2.5 ml, 5 ml, and 10 ml of water for injection, respectively, contains approximately 100 IU/ml of human coagulation factor VIII.
Beriate 2000 should be reconstituted with 10 ml of water for injection, and the resulting solution contains approximately 200 IU/ml of human coagulation factor VIII.
Other ingredients are:
Glycine, calcium chloride, sodium hydroxide (in small amounts) to adjust pH, sucrose, sodium chloride. Solvent:water for injection 2.5 ml, 5 ml, and 10 ml, respectively.
What Beriate looks like and contents of the pack
Beriate is a white powder and is supplied with water for injection.
The reconstituted solution should be clear to slightly opalescent, i.e. it may shimmer when held in light, but it must not contain any visible particles.
Pack sizes:
Pack of 250 IU containing:
1 vial of powder
1 vial of water for injection 2.5 ml
1 Mix2Vial transfer system with filter
Administration set (inner packaging):
1 single-use syringe 5 ml
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster
Pack of 500 IU containing:
1 vial of powder
1 vial of water for injection 5 ml
1 Mix2Vial transfer system with filter
Administration set (inner packaging):
1 single-use syringe 5 ml
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster.
Pack of 1000 IU containing:
1 vial of powder
1 vial of water for injection 10 ml
1 Mix2Vial transfer system with filter
Administration set (inner packaging):
1 single-use syringe 10 ml
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster.
Pack of 2000 IU containing:
1 vial of powder
1 vial of water for injection 10 ml
1 Mix2Vial transfer system with filter
Administration set (inner packaging):
1 single-use syringe 10 ml
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
CSL Behring GmbH
Emil-von-Behring-Str. 76
35041 Marburg
Germany
This medicinal product is authorised in the Member States of the European Economic Area under the following names
Austria:
Beriate 100 IU/ml Powder and solvent for solution for injection or infusion (250 IU, 500 IU, 1000 IU)
Beriate 200 IU/ml Powder and solvent for solution for injection or infusion (2000 IU)
Bulgaria:
Beriate 250 IU Powder and solvent for solution for injection or infusion
Beriate 500 IU Powder and solvent for solution for injection or infusion
Beriate 1000 IU Powder and solvent for solution for injection or infusion
Beriate 2000 IU Powder and solvent for solution for injection or infusion
Croatia:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Czech Republic:
Beriate 250 IU, Beriate 500 IU, Beriate 1000 IU, Beriate 2000 IU
Estonia:
Beriate
Latvia:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Lithuania:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Germany:
Beriate 250, Beriate 500, Beriate 1000, Beriate 2000
Hungary:
Presentations of Beriate 250, 500 and 1000:
BERIATE 100 NE/ml powder and solvent for solution for injection or infusion (250 NE, 500 NE, 1000 NE)
Presentation 2000:
BERIATE 200 NE/ml powder and solvent for solution for injection or infusion (2000 NE)
Italy:
Beriate
Poland:
Beriate 250
Beriate 500
Beriate 1000
Beriate 2000
Portugal:
Beriate
Romania:
Beriate 250 powder and solvent for solution for injection or infusion
Beriate 500 powder and solvent for solution for injection or infusion
Beriate 1000 powder and solvent for solution for injection or infusion
Beriate 2000 powder and solvent for solution for injection or infusion
Spain:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Slovakia:
Beriate 250 IU
Beriate 500 IU
Beriate 1000 IU
Beriate 2000 IU
Slovenia:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Date of last revision of the leaflet: September 2021
Information intended for healthcare professionals only:
Dosage
Monitoring of treatment
The levels of factor VIII should be properly determined during treatment to measure the appropriate dose to be administered to the patient and the frequency of repeated infusions.
Patient responses to factor VIII may vary, taking into account different recovery levels and half-lives. Dosing based on body weight may require adjustment in patients who are overweight or underweight. In particular, in the case of major surgical procedures, it is necessary to closely monitor substitution therapy by controlling the coagulation process (factor VIII activity level in plasma).
Patients should be monitored for the development of factor VIII inhibitors. See also section 2.
The number of factor VIII units administered to the patient is expressed in international units (IU), which refer to the current WHO standards for factor VIII concentrates. Factor VIII coagulant activity in plasma is expressed as a percentage (relative to normal human plasma) or preferably in IU (relative to the international standard for factor VIII in plasma).
One international unit of factor VIII activity is equivalent to the activity of factor VIII contained in 1 ml of normal human plasma.
On-demand treatment
The calculation of the required dose of factor VIII is based on the empirical finding that 1 IU of factor VIII per kg of body weight increases the activity of factor VIII in plasma by about 2% of normal activity (2 IU/dl). The required dose is calculated using the following formula:
Required dose (in IU) = body weight (in kg) x desired increase in factor VIII level (in % or IU/dl) x 0.5.
The dose of the product and the frequency of administration should always be individually tailored, depending on the clinical efficacy in individual patients.
In the following cases of bleeding, factor VIII activity should not fall below the specified values for activity in plasma (as a percentage of normal or IU/dl) during the corresponding period. The following table shows possible dosing in cases of bleeding and surgical procedures:
| Type of bleeding/surgical procedure | Required factor VIII level (% or IU/dl) | Dosing frequency (hours) / duration of treatment (days) |
| Bleeding | ||
| Mild bleeding into joints, muscle bleeding, or bleeding from the mouth | 20-40 | Repeat every 12 to 24 hours, for at least 1 day, until pain and bleeding have stopped or the bleeding has healed. |
| More extensive bleeding into joints, muscle bleeding, or haematoma | 30-60 | Repeat infusion every 12-24 hours for 3 to 4 days or more, until pain and acute dysfunction have stopped. |
| Life-threatening bleeding | 60-100 | Repeat infusion every 8 to 24 hours, until the risk has stopped. |
| Surgical procedures | ||
| Minor surgical procedures, including tooth extraction | 30-60 | Every 24 hours, for at least 1 day, until healing. |
| Major surgical procedures | 80-100 (pre- and post-operative) | Repeat infusion every 8-24 hours until proper wound healing, then therapeutically for at least 7 days to maintain factor VIII activity at 30-60% (30-60 IU/dl, equivalent to 0.30-0.60 IU/ml). |
Prophylaxis
In long-term prophylactic treatment of bleeding in patients with severe haemophilia type A, 20 to 40 IU of factor VIII per kilogram of body weight are usually administered at intervals of 2 to 3 days. In some cases, especially in younger patients, more frequent administration of factor VIII or the use of higher doses may be necessary.
Children and adolescents
Dosing in children is based on body weight, using the same principle as for adults. The frequency of administration should always be individually tailored, depending on clinical efficacy. Cases of treatment of children under the age of 6 have been reported.
Information on the pharmacological properties of VWF
In addition to its role as a protective protein for factor VIII, von Willebrand factor acts as a mediator of platelet adhesion to the site of vascular injury and plays a role in platelet aggregation.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterCSL Behring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Beriate 500Dosage form: Powder, 1000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 2000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 250 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription required
Alternatives to Beriate 500 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Beriate 500 in Spain
Alternative to Beriate 500 in Ukraine
Online doctors for Beriate 500
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Beriate 500 – subject to medical assessment and local rules.














