
OCREVUS 300 mg concentrate for infusion solution

How to use OCREVUS 300 mg concentrate for infusion solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ocrevus 300mg concentrate for solution for infusion
ocrelizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Ocrevus and what is it used for
- What you need to know before you use Ocrevus
- How to use Ocrevus
- Possible side effects
- Storage of Ocrevus
- Contents of the pack and other information
1. What is Ocrevus and what is it used for
What is Ocrevus
Ocrevus contains the active substance “ocrelizumab”. It is a type of protein called a “monoclonal antibody”. Antibodies work by binding to specific targets in your body.
What Ocrevus is used for
Ocrevus is used to treat adults with:
- Relapsing forms of multiple sclerosis (RMS)
- Primary progressive multiple sclerosis (PPMS) with early disease
What is Multiple Sclerosis:
Multiple sclerosis (MS) affects the central nervous system, especially the nerves of the brain and spinal cord. In MS, the immune system (the body's defense system) works incorrectly, attacking the protective covering (called myelin sheath) surrounding nerve cells and causing inflammation. The damage to the myelin sheath disrupts the nerves' ability to function properly.
The symptoms of MS depend on which part of the central nervous system is affected and can include problems with walking and balance, weakness, numbness, double vision and blurred vision, poor coordination, and bladder problems.
- In relapsing forms of MSpatients experience repeated episodes of symptoms (relapses). Symptoms can appear suddenly over a few hours, or slowly over several days. Symptoms disappear or improve between each relapse, but damage can build up and cause permanent disability.
- In patients with primary progressive MSsymptoms generally worsen continuously from the start of the disease.
How Ocrevus works
Ocrevus binds to specific B lymphocytes, which are a type of white blood cell that is part of the immune system and plays a role in MS. Ocrevus binds to and eliminates these specific B lymphocytes. This reduces inflammation and attacks on the myelin sheath, reduces the likelihood of experiencing a relapse, and slows down the progression of the disease.
- In relapsing forms of MS (RMS), Ocrevus helps to significantly reduce the number of relapses and significantly slow down the progression of the disease. Ocrevus also significantly increases the likelihood that a patient will not show evidence of disease activity (brain lesions, relapses, and disability worsening).
- In primary progressive MS (PPMS), Ocrevus helps to slow down the progression of the disease and reduce the deterioration of walking speed.
2. What you need to know before you use Ocrevus
Do not use Ocrevus:
- if you are allergic to ocrelizumab or any of the other ingredients of this medicine (listed in section 6).
- if you currently have an infection.
- if you have been diagnosed with serious problems with your immune system.
- if you have cancer.
If you are not sure, talk to your doctor before using Ocrevus.
Warnings and precautions
Talk to your doctor before you start using Ocrevusif any of the following conditions apply to you. Your doctor may decide to delay your treatment with Ocrevus or decide that you cannot use Ocrevus if:
- you have an infection. Your doctor will wait until the infection is resolved before giving you Ocrevus.
- you have ever had hepatitis Bor are a carrier of the hepatitis B virus. This is because medicines like Ocrevus may cause the hepatitis B virus to become active again. Before treatment with Ocrevus, your doctor will check if you are at risk of hepatitis B infection. Patients who have had hepatitis B or are carriers of the hepatitis B virus will have a blood test and will be monitored by a doctor for signs of hepatitis B infection.
- you have canceror have had cancer in the past. Your doctor may decide to delay your treatment with Ocrevus.
Effects on the immune system:
- Diseases that affect your immune system:if you have another disease that affects the immune system. You may not be suitable for treatment with Ocrevus.
- Medicines that affect your immune system:if you have ever taken, are taking, or are planned to take medicines that affect the immune system – such as chemotherapy, immunosuppressants, or other medicines used to treat MS. Your doctor may decide to delay your treatment with Ocrevus or ask you to stop taking these medicines before starting treatment with Ocrevus. For more information, see “Other medicines and Ocrevus” below.
Infusion-related reactions
- Infusion-related reactions are the most common side effect of treatment with Ocrevus.
- Tell your doctor or nurse immediately if you have any infusion-related reaction(see section 4 for a list of infusion-related reactions). Infusion-related reactions can occur during or up to 24 hours after the infusion.
- To reduce the risk of infusion-related reactions, your doctor will give you other medicines before each Ocrevus infusion (see section 3) and you will be closely monitored during the infusion and for at least 1 hour after the infusion.
Infections
- Tell your doctor before you receive treatment with Ocrevus if you think you may have an infection. Your doctor will wait until the infection is resolved before giving you Ocrevus.
- You may be more likely to get infections with Ocrevus. This is because the immune cells that Ocrevus acts on also help to fight infections.
- Before you start treatment with Ocrevus and before each subsequent infusion, your doctor may ask for a blood test to check the status of your immune system, as infections can occur more frequently in people with serious problems with their immune system.
- If you have been treated with Ocrevus for primary progressive multiple sclerosis and have difficulty swallowing, Ocrevus may increase the risk of serious pneumonia.
- Tell your doctor or nurse immediately if you experience any of these signs of infection during or after treatment with Ocrevus:
- fever or chills
- cough that does not go away
- herpes (such as cold sores, shingles, or genital ulcers).
- Tell your doctor or nurse immediately if you think your MS is getting worse or if you notice any new symptoms. This is due to a very rare and potentially life-threatening brain infection called “progressive multifocal leukoencephalopathy” (PML), which can cause symptoms similar to those of MS. PML can occur in patients taking Ocrevus.
Tell your partner or carerabout your treatment with Ocrevus. They may notice symptoms of PML that you do not notice, such as memory problems, difficulty thinking, difficulty walking, loss of vision, or changes in your speech. Your doctor may need to examine them.
Vaccines
- Tell your doctor if you have recently received any vaccine or may receive a vaccine in the near future.
- During treatment with Ocrevus, you should not receive live or live attenuated vaccines (e.g. BCG for tuberculosis or yellow fever vaccine).
- Your doctor may recommend that you receive a flu vaccine.
- Your doctor will check if you need any vaccines before starting treatment with Ocrevus. Vaccines should be given at least 6 weeks before starting treatment with Ocrevus.
Children and adolescents
Ocrevus is not intended for use in children and adolescents under 18 years of age. This is because it has not been studied in this age group.
Other medicines and Ocrevus
Tell your doctor if you are using, have recently used, or might use any other medicines.
Especially, tell your doctor if:
- you have ever taken, are taking, or are planned to take medicines that affect the immune system– such as chemotherapy, immunosuppressants, or other medicines used to treat MS. The effect on the immune system of these medicines given with Ocrevus may be too strong. Your doctor may decide to delay your treatment with Ocrevus or ask you to stop taking these medicines before starting treatment with Ocrevus.
- you are taking medicines for high blood pressure. This is because Ocrevus may lower your blood pressure. Your doctor may ask you to stop taking your blood pressure medicines 12 hours before each Ocrevus infusion.
If any of these conditions apply to you (or you are not sure), talk to your doctor before using Ocrevus.
Pregnancy
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, ask your doctor for advice before taking this medicine. This is because Ocrevus may cross the placenta and affect your baby.
- Do not use Ocrevus if you are pregnant unless you have discussed it with your doctor. Your doctor will weigh the benefits of using Ocrevus against the risks to your baby.
- Ask your doctor for advice before vaccinating your baby.
Contraception for women
If you are able to become pregnant (are of childbearing potential), women should use effective contraception:
- during treatment with Ocrevus and
- for 4 months after the last infusion of Ocrevus.
Breastfeeding
Do not breastfeed while receiving treatment with Ocrevus. This is because Ocrevus may pass into breast milk.
Driving and using machines
It is not known if Ocrevus can affect your ability to drive or use tools or machines.
Your doctor will tell you if MS can affect your ability to drive or use tools or machines safely.
Ocrevus contains sodium
This medicine contains less than 1 mmol of sodium(23 mg) per dose, which is essentially “sodium-free”.
3. How to use Ocrevus
Ocrevus will be given to you by a doctor or nurse experienced in the use of this treatment.
You will be kept under observation during the administration of the medicine in case you experience any side effects. Ocrevus will always be given as a drip (intravenous infusion).
Medicines you will receive before Ocrevus
Before you receive Ocrevus, you will be given other medicines to prevent or reduce possible side effects such as infusion-related reactions (see sections 2 and 4 for more information on infusion-related reactions).
You will receive a corticosteroid and an antihistamine before each infusion and may also be given medicines to reduce fever.
How much Ocrevus and how often it is given
You will receive a total dose of 600 mg of Ocrevus every 6 months.
- The first dose of 600 mg of Ocrevus will be given as 2 infusions (300 mg each) separated by a 2-week interval. Each infusion will take around 2 hours and 30 minutes.
- Subsequent doses of 600 mg of Ocrevus will be given as a single infusion. Depending on the rate of the next infusion, it will take either around 3 hours and 30 minutes or 2 hours.
How Ocrevus is given
- Ocrevus will be given by a doctor or nurse. It will be given as an infusion into a vein (intravenous infusion or “IV” infusion).
- You will be closely monitored while you receive Ocrevus and for at least 1 hour after the infusion. This is in case you experience any side effects such as infusion-related reactions. The infusion may be slowed down, temporarily stopped, or permanently stopped if you experience an infusion-related reaction, depending on the severity of the reaction (see sections 2 and 4 for more information on infusion-related reactions).
If you miss an infusion of Ocrevus
- If you miss an infusion of Ocrevus, talk to your doctor to schedule a new infusion as soon as possible. Do not wait until your next scheduled infusion.
- To get the full benefit of Ocrevus, it is important that you receive each infusion when it is due.
If you stop treatment with Ocrevus
- It is important that you continue your treatment for as long as you and your doctor think it is helping you.
- Some side effects may be related to low levels of B lymphocytes. After you finish treatment with Ocrevus, you may continue to experience side effects until your B lymphocytes return to normal levels. Your B lymphocyte levels in the blood will gradually increase to normal levels. This may take between 6 months and 2.5 years, or even longer in rare cases.
- Before you start taking any other medicine, tell your doctor when you last received an infusion of Ocrevus.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported with Ocrevus:
Serious side effects:
Infusion-related reactions
- Infusion-related reactions are the most common side effect of treatment with Ocrevus (very common: may affect more than 1 in 10 people). In most cases, they are mild, but some can be serious.
- Tell your doctor or nurse immediately if you experience any signs or symptoms of an infusion-related reaction during or up to 24 hours after the infusion.Symptoms can include, among others:
- itching of the skin
- rash
- hives
- redness of the skin
- irritation or pain in the throat
- difficulty breathing
- swelling of the throat
- flushing
- low blood pressure
- fever
- fatigue
- headache
- dizziness
- nausea
- fast heartbeat.
- If you experience an infusion-related reaction, you will be given medicines to treat it, and the infusion may need to be slowed down or stopped. When the reaction has resolved, the infusion can be continued. If the infusion-related reaction is life-threatening, your doctor will permanently stop treatment with Ocrevus.
Infections
- You may be more likely to get infections with Ocrevus. The following infections have been seen in patients treated with Ocrevus in the context of MS:
- Very common: may affect more than 1 in 10 people
- throat pain and nasal discharge (upper respiratory tract infection)
- flu.
- Common: may affect up to 1 in 10 people
- sinus infection
- bronchitis (inflammation of the bronchial tube)
- herpes infection (such as cold sores, shingles, or genital ulcers)
- stomach and intestine infection (gastroenteritis)
- respiratory tract infection
- viral infection
- skin infection (cellulitis)
Some of these can be serious.
- Tell your doctor or nurse immediately if you experience any of these signs of infection:
- fever or chills
- cough that does not go away
- herpes (such as cold sores, shingles, or genital ulcers).
Other side effects
Very common:may affect more than 1 in 10 people
- decrease in certain proteins in the blood (immunoglobulins) that help protect against infections.
Common:may affect up to 1 in 10 people
- eye discharge with itching, redness, and swelling (conjunctivitis)
- cough
- thick mucus in the nose, throat, or chest
- low levels of a type of white blood cell (neutropenia).
Unknown: frequency cannot be estimated from the available data
- delayed reduction in white blood cells
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ocrevus
Healthcare professionals at the hospital or clinic will store Ocrevus under the following conditions:
- Keep this medicine out of sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the outer packaging and on the label of the vial after “EXP”. The expiry date refers to the last day of the month stated.
- Store this medicine in a refrigerator (2°C - 8°C). Do not freeze. Keep the vials in the outer packaging to protect them from light.
Ocrevus must be diluted before administration. The dilution will be performed by a healthcare professional. It is recommended to use the medicine immediately after dilution. If not used immediately, the in-use storage times and conditions prior to use will be the responsibility of the healthcare professional and will normally not exceed 24 hours at 2°C – 8°C and 8 hours at room temperature.
Medicines should not be disposed of via wastewater or household waste. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Ocrevus
- The active substance is ocrelizumab. Each vial contains 300 mg of ocrelizumab in 10 ml at a concentration of 30 mg/ml.
- The other ingredients are sodium acetate trihydrate (see Section 2 “Ocrevus contains sodium”), glacial acetic acid, trehalose dihydrate, polysorbate 20, and water for injectable preparations.
Appearance and Package Contents of the Product
- Ocrevus is a solution that is between transparent and slightly opalescent, and between colorless and light brown.
- It is supplied as a concentrate for solution for infusion.
- This medicine is available in packs containing 1 or 2 vials (10 ml concentrate vials). Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
D-79639 Grenzach-Wyhlen
Germany
For further information, please contact the local representative of the Marketing Authorisation Holder:
België/Belgique/Belgien N.V. Roche S.A. Tél/Tel: +32 (0) 2 525 82 11 | Lietuva UAB “Roche Lietuva” Tel.: +370 5 2546799 |
| Luxembourg/Luxemburg (See Belgium) |
Ceská republika Roche s. r. o. Tel.: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel.: +36 - 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel.: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel.: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Norge Roche Norge AS Tel.: +47 - 22 78 90 00 |
Ελλάδα Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel.: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel.: +385 1 4722 333 | România Roche România S.R.L. Tel.: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Italia Roche S.p.A. Tel.: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Kύπρος Γ.Α.Σταμής & Σια Λτδ. Tel.: +357 - 22 76 62 76 | Sverige Roche AB Tel.: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel.: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu.This information is intended only for healthcare professionals:
For more information, read the Summary of Product Characteristics.
To improve the traceability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
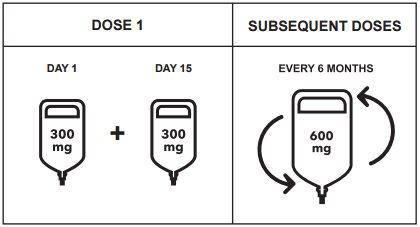
Posology
- Initial dose
The initial dose of 600 mg is administered as two separate intravenous infusions; the first infusion of 300 mg followed by a second infusion of 300 mg administered 2 weeks later.
- Subsequent doses
After the initial dose, subsequent doses of ocrelizumab are administered as a single intravenous infusion of 600 mg every 6 months (Table 1). The first of the subsequent 600 mg doses should be administered 6 months after the first infusion of the initial dose. A minimum interval of 5 months between each ocrelizumab dose must be respected.
Figure 1: Dosing and Administration Schedule of Ocrevus
Management of IRs before Infusion
- Treatment with ocrelizumab should be initiated and supervised by an experienced healthcare professional with access to emergency medical services for the management of serious reactions such as IRs, hypersensitivity reactions, and/or anaphylactic reactions.
- Premedication for IRs
The following premedications will be administered before each Ocrevus infusion to reduce the frequency and severity of IRs:
- 100 mg of methylprednisolone intravenously (or equivalent) approximately 30 minutes before each infusion;
- an antihistamine approximately 30-60 minutes before each infusion.
In addition, premedication with an antipyretic (e.g., paracetamol) may also be considered approximately 30-60 minutes before each Ocrevus infusion.
- Hypotension may occur as a symptom of an IR during Ocrevus infusions. Therefore, consideration should be given to withholding antihypertensive treatment for 12 hours before and during each Ocrevus infusion. Patients with a history of congestive heart failure (New York Heart Association Classes III and IV) have not been studied.
Instructions for Dilution
- The product should be prepared by a healthcare professional using aseptic techniques. Do not shake the vial. A sterile needle and syringe should be used to prepare the diluted infusion solution.
- The medicine is for single use only.
- The concentrate may contain fine translucent and/or reflective particles associated with increased opalescence. Do not use the concentrate if it appears discolored or if the concentrate contains any foreign particles.
- The medicine must be diluted prior to administration. Intravenous infusion solutions are prepared by diluting the concentrate in an infusion bag containing 9 mg/ml of 0.9% isotonic sodium chloride solution (300 mg/250 ml or 600 mg/500 ml), to achieve a final concentration of approximately 1.2 mg/ml. The diluted infusion solution should be administered through an infusion set with an in-line filter with a pore size of 0.2 or 0.22 microns.
- Prior to starting the intravenous infusion, the contents of the infusion bag should be at room temperature to avoid a reaction to the infusion due to the administration of the solution at low temperatures.
Method of Administration
- After dilution, the treatment is administered as an intravenous infusion through a dedicated line.
- Infusions should not be administered by rapid intravenous injection or bolus.
Table 1: Dosing and Administration Schedule of Ocrevus
Amount of ocrelizumab to be administered | Infusion Instructions | ||
Initial dose (600 mg) divided into 2 infusions | Infusion 1 | 300 mg in 250 ml |
|
Infusion 2 (2 weeks later) | 300 mg in 250 ml | ||
Subsequent doses (600 mg) single infusionevery 6 months | Option 1 Infusion of approximately 3.5 hours duration | 600 mg in 500 ml |
|
Or | |||
Option 2 Infusion of approximately 2 hours duration | 600 mg in 500 ml |
|
Management of IRs during and after Infusion
Patient should be monitored during the infusion and for at least one hour after completion of the infusion.
During Infusion
- Infusion adjustments in case of IRs
In case of IRs during infusion, refer to the following adjustments.
Potentially life-threatening IRs
If signs of a potentially life-threatening or debilitating IR occur during an infusion, such as acute hypersensitivity or acute respiratory distress syndrome, the infusion should be stopped immediately and the patient should receive appropriate treatment. In these patients, the infusion should be permanently discontinued (see section 4.3).
Severe IRs
If a patient experiences a severe IR (such as dyspnea) or a combination of symptoms of flushing, fever, and throat irritation, the infusion should be interrupted immediately and the patient should receive symptomatic treatment. The infusion should be restarted only after resolution of all symptoms. The initial infusion rate at the time of restart should be half of the infusion rate at the time of the reaction. No adjustment of the infusion is required for subsequent infusions unless the patient experiences an IR.
Mild to moderate IRs
If a patient experiences a mild to moderate IR (e.g., headache), the infusion rate should be reduced to half of the infusion rate at the time of the event. This reduced rate should be maintained for at least 30 minutes. If tolerated, the infusion rate may be increased according to the patient's initial infusion rate. No adjustment of the infusion is required for subsequent infusions unless the patient experiences an IR.
- Patients who experience severe pulmonary symptoms, such as bronchospasm or asthma exacerbation, should have the infusion permanently discontinued. After administering symptomatic treatment, the patient should be monitored until resolution of pulmonary symptoms, as initial improvement of clinical symptoms may be followed by deterioration.
- Hypersensitivity may be clinically indistinguishable from an IR in terms of symptoms. If a hypersensitivity reaction is suspected during infusion, the infusion should be permanently discontinued.
After Infusion
- Patient should be monitored for at least one hour after completion of the infusion to detect any symptoms of an IR.
- Physicians should inform patients that an IR can occur within 24 hours after infusion.
Shelf Life
Unopened vial
2 years
Diluted infusion solution for intravenous infusion
- Chemical and physical stability has been demonstrated for 24 hours at 2-8°C and subsequently for 8 hours at room temperature.
- From a microbiological point of view, the infusion solution should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and would normally not exceed 24 hours at 2-8°C and subsequently 8 hours at room temperature, unless the dilution is performed under controlled and validated aseptic conditions.
- In case an intravenous infusion cannot be completed on the same day, the remaining solution should be discarded.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OCREVUS 300 mg concentrate for infusion solutionDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for OCREVUS 300 mg concentrate for infusion solution
Discuss questions about OCREVUS 300 mg concentrate for infusion solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







