
NUMETA G13% Emulsion for Infusion


How to use NUMETA G13% Emulsion for Infusion
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NUMETA G13%E emulsion for infusion
Read all of this leaflet carefully before your child starts using this medicine because it contains important information for them.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your child's doctor, pharmacist, or nurse.
- If your child experiences any side effects, talk to your child's doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Numeta G13%E and what is it used for
- What you need to know before Numeta G13%E is administered to your child
- How Numeta G13%E will be administered
- Possible side effects
- Storage of Numeta G13%E
- Contents of the pack and further information
1. What is Numeta G13%E and what is it used for
Numeta G13%E is a specialized nutrition emulsion designed for premature newborns. It is administered through a tube connected to your child's vein when they are unable to eat all their food by mouth.
Numeta comes in a three-compartment bag with three independent chambers containing:
a 50% glucose solution
a 5.9% pediatric amino acid solution with electrolytes
a 12.5% lipid (fat) emulsion
Depending on your child's needs, two or three of these solutions are mixed in the bag before being administered to your child.
Numeta G13%E should only be used under medical supervision.
2. What you need to know before Numeta G13%E is administered to your child
Numeta G13%Emust not be administered to your child in the following cases:
With the glucose and amino acid/electrolyte solutions mixed in the bag ("2 in 1"):
- If your child is allergic to eggs, soy, peanuts, or any of the ingredients of this medicine or component of the packaging (listed in section 6).
- If your child's body has problems using the constituents of proteins.
- If your child has high concentrations of any of the electrolytes included in Numeta G13%E in their blood.
- Numeta G13%E (or other solutions containing calcium) must not be administered at the same time as the antibiotic ceftriaxone, even if different infusion lines or sites are used. There is a risk of particle formation in the newborn's bloodstream, which can be fatal.
- If your child has hyperglycemia (especially high blood sugar levels).
With the glucose, amino acid/electrolyte, and lipid solutions mixed in the bag ("3 in 1").
All the situations mentioned for "2 in 1", plus the following:
- If your child has a particularly high level of fat in their blood.
In all cases, the doctor will decide whether to administer this medicine to your child based on factors such as age, weight, and clinical condition. They will also take into account the results of all tests performed.
Warnings and precautions
Talk to your child's doctor or nurse before Numeta G13%E is administered.
When used in newborns and children under 2 years, the emulsion (in the bags and administration equipment) must be protected from light exposure until administration is complete. Exposure of Numeta G13%E to ambient light, especially after mixing with oligoelements or vitamins, generates peroxides and other degradation products that can be reduced by protecting the product from light exposure.
Allergic reactions:
Infusion must be stopped immediately if any signs or symptoms of an allergic reaction appear (such as fever, sweating, chills, headache, skin rash, or difficulty breathing). This medicine contains soybean oil, which can rarely cause hypersensitivity reactions. In rare cases, it has been observed that some people who are allergic to peanut proteins are also allergic to soybean proteins.
Numeta G13%E contains glucose from cornstarch, so it should be used with caution in patients with known allergy to corn or its products.
Risk of particle formation with ceftriaxone (antibiotic):
This medicine must not be mixed or administered at the same time as the antibiotic ceftriaxone, even if different infusion lines are used. There is a risk of particle formation in the bloodstream, which can be fatal.
Your doctor is aware of this and will not administer them together, even through different lines or infusion sites.
Formation of small particles in the blood vessels of the lungs:
Difficulty breathing can also be a sign of the formation of small particles that block the blood vessels of the lungs (pulmonary vascular precipitates). If your child experiences difficulty breathing, inform your child's doctor or nurse. They will decide on the measures to be taken.
Infection and sepsis:
The doctor will closely monitor your child for any symptoms of infection. The use of an "aseptic technique" (germ-free technique) when placing and maintaining the catheter, as well as when preparing the nutrition formula, can reduce the risk of infection.
Occasionally, children may develop infections and sepsis (bacteria in the blood) when they have a tube connected to a vein (intravenous catheter). Certain medications and diseases can increase the risk of developing an infection or sepsis. Patients who require parenteral nutrition (nutrition administered through a tube connected to a vein) are more likely to develop an infection due to their clinical condition.
Fat overload syndrome:
Cases of fat overload syndrome have been reported with similar products. A reduction or limitation of the body's ability to eliminate the fats contained in Numeta G13%E, or an overdose, can cause a "fat overload syndrome" (see sections 3 and 4).
Changes in blood chemical levels:
The doctor will check and review your child's fluids, blood chemicals, and other blood values during treatment with Numeta G13%E. Occasionally, refeeding someone who is severely malnourished can lead to changes in blood chemical levels that may need to be corrected. Excess fluid in the tissues and swelling can also occur. It is recommended to start parenteral nutrition slowly and under supervision.
Monitoring and adjustment
The doctor will closely monitor and adjust the dose of Numeta G13%E to your child's individual needs, especially if they have the following conditions:
- Severe post-traumatic states.
- Severe diabetes mellitus.
- Shock.
- Heart attack.
- Severe infection.
- Certain types of coma.
Use with caution:
Numeta G13%E should be used with caution if your child has:
- Pulmonary edema (fluid in the lungs) or heart failure.
- Severe liver problems.
- Problems absorbing nutrients.
- High blood sugar levels.
- Kidney problems.
- Severe metabolic disorders (when the body cannot break down substances normally).
- Blood coagulation disorders.
The levels of fluids in your child's body, liver analysis values, and blood values will be carefully monitored.
There is limited data on the administration of this medicine in premature infants under 28 weeks of gestational age.
Use of Numeta G13%E with other medicines
Tell your child's doctor if they are taking or have recently taken any other medicines.
Numeta G13%Emust not be administered at the same time as:
- Ceftriaxone(an antibiotic), even if different infusion lines are used, due to the risk of particle formation.
- Bloodthrough the same infusion line due to the risk of pseudoagglutination (red blood cells become stacked).
- Ampicillin, phenytoin, and furosemidethrough the same infusion line, as there is a risk of particle formation.
Coumarin and warfarin (anticoagulants)
The doctor will closely monitor your child if they are taking coumarin or warfarin. These medicines are anticoagulants used to prevent blood clotting. Olive and soybean oil naturally contain vitamin K1. Vitamin K1 can interfere with the action of medicines like coumarin and warfarin.
Laboratory tests
The lipids included in this emulsion can interfere with the results of certain laboratory tests. Laboratory tests can be performed once 5 to 6 hours have passed since the administration of lipids or if no more lipids are administered.
Interaction of Numeta with medicines that may affect potassium levels/metabolism:
Numeta G13%E contains potassium. High potassium levels in the blood can cause an abnormal heart rhythm. Special attention should be paid to patients who take diuretics (medicines that reduce fluid retention), ACE inhibitors (medicines used to treat high blood pressure), or angiotensin II receptor antagonists (medicines used to treat high blood pressure) or immunosuppressants (medicines that can decrease the body's natural defenses). These types of medicines can increase potassium levels.
3. How Numeta G13%E will be administered
Your child will always be administered Numeta G13%E exactly as their doctor has indicated. Ask your child's doctor if you have any doubts.
Age group
Numeta G13%E has been designed to meet the nutritional needs of premature newborns.
Numeta G13%E may not be suitable for some premature infants, as their clinical condition may require customized formulations that meet their specific nutritional needs. The doctor will decide if this medicine is suitable for your child.
Administration
This medicine is an emulsion for infusion. It is administered through a plastic tube connected to a vein in your child's arm or a large vein in their chest.
The doctor may choose not to administer lipids to your child. The design of the Numeta G13%E bag allows the non-permanent seal between the amino acid/electrolyte and glucose chambers to be broken, if necessary. The seal between the amino acid and lipid chambers remains intact in this case. The contents of the bag can be infused without lipids.
When used in newborns and children under 2 years, the emulsion (in the bags and administration equipment) must be protected from light exposure until administration is complete (see section 2).
Dosage and duration of treatment
The doctor will decide on the dose your child needs and how long they will be administered. The dose depends on your child's nutritional needs and will be based on their weight, medical condition, and the body's ability to digest and absorb the ingredients of Numeta G13%E. Additional protein or nutrition may also be administered orally or enterally.
If your child is administered more Numeta G13%E than they should
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone 915.620.420
Symptoms
Receiving too much medicine or administering it too quickly could cause:
- Nausea (feeling unwell).
- Vomiting.
- Chills.
- Electrolyte disturbances (inappropriate amounts of electrolytes in the blood).
- Signs of hypervolemia (increased blood volume, too much fluid in the blood vessels).
- Acidosis (increased blood acidity).
In these cases, the infusion should be stopped immediately. The doctor will decide if other actions are necessary.
An overdose of the fats contained in Numeta G13%E can cause a "fat overload syndrome", which is usually reversible once the infusion is interrupted. In newborns (neonates) and small children (infants), the fat overload syndrome has been associated with respiratory disorders that cause a reduction in oxygen in the body (difficulty breathing) and conditions that cause an increase in blood acidity (acidosis).
To avoid these reactions, the doctor will regularly monitor your child's condition and analyze their blood levels during treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not all children will experience them.
If you notice any change in the way your child feels during or after treatment, tell your child's doctor or nurse immediately.
The tests that the doctor will perform on your child while they are receiving this medicine should minimize the risk of side effects.
If symptoms of an allergic reaction appear, the infusion should be stopped and the doctor contacted immediately. This can be serious and symptoms may include:
- Sweating.
- Chills.
- Headache.
- Skin rash.
- Difficulty breathing.
Other side effects that have been observed are:
Frequent: may affect up to 1 in 10 people
- Low phosphate levels in the blood (hypophosphatemia).
- High blood sugar levels (hyperglycemia).
- High calcium levels in the blood (hypercalcemia).
- High triglyceride levels in the blood (hypertriglyceridemia).
- Electrolyte disturbances (hyponatremia).
Uncommon: may affect up to 1 in 100 people
- High lipid levels in the blood (hyperlipidemia).
- A disorder in which bile cannot flow from the liver to the duodenum (cholestasis). The duodenum is part of the intestine.
Unknown: frequency cannot be estimated from available data(these side effects have been reported with Numeta G13%E and G16%E when administered peripherally with insufficient dilution).
- Skin necrosis
- Soft tissue damage
- Extravasation
The following side effects have been reported with other parenteral nutrition products:
- The reduced or limited ability to eliminate the fats contained in Numeta G13%E can lead to a "fat overload syndrome". The following signs and symptoms of this syndrome are usually reversible when the lipid emulsion infusion is stopped:
- Sudden and severe worsening of the patient's medical condition.
- High fat levels in the blood (hyperlipidemia).
- Fever.
- Fatty infiltration of the liver (hepatomegaly).
- Worsening of liver function.
- Decreased red blood cell count, which can cause pale skin and weakness or difficulty breathing (anemia).
- Low white blood cell count, which can increase the risk of infection (leucopenia).
- Low platelet count, which can increase the risk of bruising and/or bleeding (thrombocytopenia).
- Blood coagulation disorders, which affect the blood's ability to clot.
- Respiratory disorders that cause a reduction in oxygen in the body (difficulty breathing).
- Conditions that cause an increase in blood acidity (acidosis).
- A coma that requires hospitalization.
- Formation of small particles that can block the blood vessels of the lungs (pulmonary vascular precipitates) or cause difficulty breathing.
Reporting of side effects:
If your child experiences any side effects, talk to your child's doctor or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Numeta G13%E
Keep this medicine out of the sight and reach of children when not being administered.
When used in newborns and children under 2 years, the emulsion (in the bags and administration equipment) must be protected from light exposure until administration is complete (see section 2).
Do not use this medicine after the expiry date stated on the bag and outer packaging (MM/YYYY). The expiry date is the last day of the month indicated.
Do not freeze.
Store in the overbag.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Appearance ofNumeta G13%Eand Container Content
Numeta G13%E is presented in the form of a bag with three chambers. Each bag contains a sterile combination of a glucose solution, a pediatric amino acid solution with electrolytes, and a lipid emulsion, as described below:
Container Size | Glucose50% Solution | Amino Acid 5.9% Solution with Electrolytes | Lipid12.5% Emulsion |
300 ml | 80 ml | 160 ml | 60 ml |
Appearance before reconstitution:
- The amino acid and glucose chamber solutions are transparent, colorless, or slightly yellowish.
- The lipid emulsion chamber contains a uniform white milky liquid.
Appearance after reconstitution:
- The "2 in 1" perfusion solution (amino acids/electrolytes and glucose) is transparent, colorless, or slightly yellowish.
- The "3 in 1" perfusion emulsion has a uniform white milky appearance.
The three-compartment bag is a multilayer plastic bag.
To avoid contact with air, Numeta G13%E is packaged inside an oxygen barrier overbag, which also contains an oxygen absorber and an oxygen indicator.
Container Sizes
300 ml bag: 10 units per box
1 bag of 300 ml
Only some container sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Baxter, S.L.
Pouet de Camilo 2, 46394 Ribarroja del Turia (Valencia)
Manufacturer
Baxter S.A.
Boulevard Rene Branquart, 80
7860 Lessines
Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Country | Name |
Austria Germany | Numeta G 13 % E Emulsion zur Infusion |
Belgium Luxembourg | NUMETZAH G13%E, émulsion pour perfusion |
France | NUMETAH G13 %E PREMATURES, emulsion pour perfusion |
Denmark Norway Sweden | Numeta G13E |
Czech Republic | NUMETA G 13 % E |
Greece | NUMETA Preterm G 13 E |
Netherlands | NUMETA G13%E emulsie voor infusie |
Ireland Malta United Kingdom | Numeta G13%E Preterm, Emulsion for Infusion |
Italy | NUMETA G13E emulsione per infusione |
Finland | Numeta G13E infuusioneste, emulsio |
Poland | NUMETA G 13 % E Preterm |
Portugal | Numeta G13%E |
Spain | NUMETA G13%E, emulsión para perfusión |
Date of the last review of this prospectus
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------
This information is intended solely for healthcare professionals:*
- Please note that in certain cases this product may be administered at home by parents or other caregivers. In these cases, parents/caregivers should read the following information.
No medication should be added to the bag without first checking compatibility. Particles may form or the lipid emulsion may break down, which could block blood vessels.
Numeta G13%E must be at room temperature before use.
Before administering Numeta G13%E, you should prepare the bag as shown below.
Make sure the bag is not damaged and use it only if it is not damaged. An undamaged bag looks like this:
- The non-permanent seals are intact. This is observed because there is no mixture of any of the three chambers.
- The amino acid solution and the glucose solution are transparent, colorless or slightly yellowish, and without visible particles.
- The lipid emulsion is a uniform white milky liquid.
Before opening the overbag, examine the color of the oxygen indicator.
- Compare it with the reference color printed next to the OK symbol and shown in the printed area of the indicator label.
- Do not use the product if the color of the oxygen indicator does not match the reference color printed next to the OK symbol.
Figures 1 and 2 illustrate how to remove the overbag. Discard it along with the oxygen indicator and the oxygen absorber.
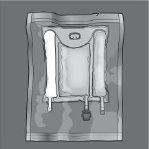
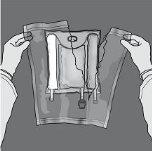
Figure 1 Figure 2
Preparation of the mixture
- Make sure the product is at room temperature before breaking the non-permanent seals.
- Place the bag on a clean and flat surface.
Activation of the 3-chamber bag (breaking of the two non-permanent seals)
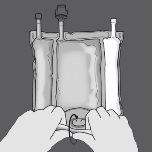
Step 1: Roll the bag from the hanger side in D
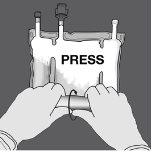
Step 2: Press until the non-permanent seals open.
Step 3: Change direction and roll the bag towards the hanger in D until the seal is completely open. Follow the same steps to open the second non-permanent seal.
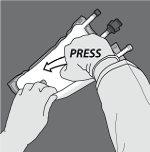
Step 4: Turn the bag at least three times to mix the contents well. The appearance of the mixed solution should be a white milky emulsion.
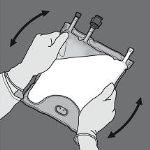
Step 5: Remove the protective cap from the administration point and insert the intravenous administration equipment.
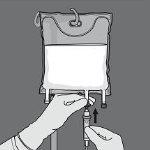
Activation of the 2-chamber bag (breaking of the non-permanent seal between the amino acid and glucose chambers)
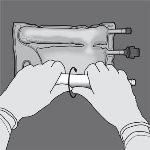
Step 1: To break only the non-permanent seal of amino acids/glucose, start rolling the bag from the corner of the hanger in D of the seal that separates the amino acid and glucose chambers and press to open the seal that separates both compartments.
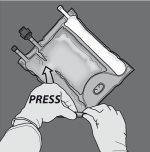
Step 2: Place the bag so that the compartment with lipid emulsion is facing the operator and roll the bag while protecting the compartment with lipid emulsion in the palms of the hands.
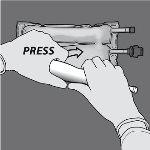
Step 3: With one hand, apply pressure by rolling the bag towards the tubes.
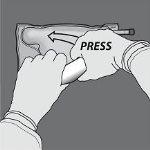
Step 4: Then, change direction and roll the bag towards the hanger in D, pressing with the other hand until the seal that separates the amino acid and glucose solutions is completely open.
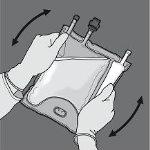
Step 5: Turn the bag at least three times to mix the contents well. The appearance of the mixed solution should be transparent, colorless or slightly yellowish.
Step 6: Remove the protective cap from the administration point and insert the intravenous administration equipment.
The administration rate should be gradually increased during the first hour, and adjusted based on the following factors:
The dose to be administered
The daily volume intake
The duration of the perfusion.
Administration method
When used in newborns and children under 2 years, the emulsion (in the bag and in the administration equipment) should be protected from light exposure until administration is completed.
The use of a 1.2 micras filter is recommended for the administration of Numeta G13%E.
Due to its high osmolarity, Numeta G13%E can only be administered undiluted through a central vein; however, a suitable dilution of Numeta G13%E with water for injectable preparations reduces the osmolarity and allows peripheral perfusion. The following formula indicates the impact of dilution on the osmolarity of the bags.
Final osmolarity | = | Bag volume x initial osmolarity |
Water added + Bag volume |
The following table shows examples of osmolarity for the additions of 2 and 3 chamber bags activated after the addition of water for injectable preparations:
Amino acids and glucose(2-chamber bag activated) | Amino acids, glucose andlipids (3-chamber bag activated) | |
Initial volume in the bag (ml) | 240 | 300 |
Initial osmolarity (mOsm/l approx) | 1400 | 1150 |
Volume of water added (ml) | 240 | 300 |
Final volume after addition (ml) | 480 | 600 |
Osmolarity after addition (mOsm/l approx) | 700 | 575 |
Medication addition
Exposure to light of intravenous parenteral nutrition solutions, especially after mixing with oligoelements or vitamins, can have adverse effects on the clinical outcome of newborns due to the generation of peroxides and other degradation products. When used in newborns and children under 2 years, Numeta G13%E should be protected from ambient light until administration is completed.
Compatible medications can be added to the reconstituted mixture (after opening the non-permanent seals and mixing the contents of the two or three chambers).
Vitamins can also be added to the glucose chamber before reconstituting the mixture (before opening the non-permanent seals and mixing the solutions and emulsion).
In Tables 1-4, the possible additions of commercially available oligoelement solutions (identified as TE1 and TE4), vitamins (identified as lyophilized V1 and emulsion V2), and electrolytes in defined quantities are shown.
1 Compatibility with TE4, V1 and V2
Table 1: Compatibility of 3-in-1 (3-chamber bag activated) with or without water dilution
Per 300 ml (3-in-1 mixture with lipids) | ||||||
Mixture without dilution | Mixture diluted | |||||
Additives | Level included | Maximum addition | Maximum total level | Level included | Maximum addition | Maximum total level |
Sodium (mmol) | 6.6 | 5.0 | 11.6 | 6.6 | 5.0 | 11.6 |
Potassium (mmol) | 6.2 | 4.2 | 10.4 | 6.2 | 4.2 | 10.4 |
Magnesium (mmol) | 0.47 | 0.83 | 1.3 | 0.47 | 0.83 | 1.3 |
Calcium (mmol) | 3.8 | 3.5 | 7.3 | 3.8 | 3.5 | 7.3 |
Phosphate* (mmol) | 3.8 | 2.5 | 6.3 | 3.8 | 2.5 | 6.3 |
Oligoelements and vitamins | - | 15 ml TE4+ 1.5 vial V1+ 25 ml V2 | 15 ml TE4+ 1.5 vial V1+ 25 ml V2 | - | 15 ml TE4+ 1.5 vial V1+ 25 ml V2 | 15 ml TE4+ 1.5 vial V1+ 25 ml V2 |
Water for injectable preparations | - | - | - | - | 300 ml | 300 ml |
- Organic phosphate
Table 2: Compatibility of 2-in-1 (2-chamber bag activated) with or without water dilution
Per 240 ml (2-in-1 mixture without lipids) | ||||||
Mixture without dilution | Mixture diluted | |||||
Additive | Level included | Maximum addition | Maximum total level | Level included | Maximum addition | Maximum total level |
Sodium (mmol) | 6.4 | 17.6 | 24 | 6.4 | 0.0 | 6.4 |
Potassium (mmol) | 6.2 | 17.8 | 24 | 6.2 | 0.0 | 6.2 |
Magnesium (mmol) | 0.47 | 2.13 | 2.6 | 0.47 | 0.0 | 0.47 |
Calcium (mmol) | 3.8 | 3.5 | 7.3 | 3.8 | 0.0 | 3.8 |
Phosphate* (mmol) | 3.2 | 4.0 | 7.2 | 3.2 | 0.0 | 3.2 |
Oligoelements and vitamins | - | 2.5 ml TE4+ ¼ vial V1 | 2.5 ml TE4+ ¼ vial V1 | - | 2.5 ml TE4+ ¼ vial V1 | 2.5 ml (¼ vial) TE4+ ¼ vial V1 |
Water for injectable preparations | - | - | - | - | 240 ml | 240 ml |
- Organic phosphate
2 Compatibility with TE1, V1 and V2
Table 3: Compatibility of 3-in-1 (3-chamber bag activated) with or without water dilution
Per 300 ml (3-in-1 mixture with lipids) | ||||||
Mixture without dilution | Mixture diluted | |||||
Additives | Level included | Maximum addition | Maximum total level | Level included | Maximum addition | Maximum total level |
Sodium (mmol) | 6.6 | 5.0 | 11.6 | 6.6 | 0.0 | 6.6 |
Potassium (mmol) | 6.2 | 4.2 | 10.4 | 6.2 | 0.0 | 6.2 |
Magnesium (mmol) | 0.47 | 0.83 | 1.3 | 0.47 | 0.0 | 0.47 |
Calcium (mmol) | 3.8 | 1.9 | 5.7 | 3.8 | 0.0 | 3.8 |
Phosphate* (mmol) | 3.8 | 2.5 | 6.3 | 3.8 | 0.0 | 3.8 |
Oligoelements and vitamins | - | 2.5 ml TE1+ ¼ vial V1+ 2.5 ml V2 | 2.5 ml TE1+ ¼ vial V1+ 2.5 ml V2 | - | 2.5 ml TE1+ ¼ vial V1+ 2.5 ml V2 | 2.5 ml TE1+ ¼ vial V1+ 2.5 ml V2 |
Water for injectable preparations | - | - | - | - | 300 ml | 300 ml |
- Organic phosphate
Table 4: Compatibility of 2-in-1 (2-chamber bag activated) with or without water dilution
Per 240 ml (2-in-1 mixture without lipids) | ||||||
Mixture without dilution | Mixture diluted | |||||
Additives | Level included | Maximum addition | Maximum total level | Level included | Maximum addition | Maximum total level |
Sodium (mmol) | 6.4 | 17.6 | 24 | 6.4 | 0.0 | 6.4 |
Potassium (mmol) | 6.2 | 17.8 | 24 | 6.2 | 0.0 | 6.2 |
Magnesium (mmol) | 0.47 | 2.13 | 2.6 | 0.47 | 0.0 | 0.47 |
Calcium (mmol) | 3.8 | 3.5 | 7.3 | 3.8 | 0.0 | 3.8 |
Phosphate* (mmol) | 3.2 | 4.0 | 7.2 | 3.2 | 0.0 | 3.2 |
Oligoelements and vitamins | - | 2.5 mL TE1+ ¼ vial V1 | 2.5 mL TE1+ ¼ vial V1 | - | 2.5 mL TE1+ ¼ vial V1 | 2.5 mL (¼ vial TE1+ ¼ vial V1 |
Water for injectable preparations | - | - | - | - | 240 ml | 240 ml |
- Organic phosphate
The composition of the vitamin and oligoelement preparations is illustrated below in Tables 5 and 6:
Table 5: Composition of the commercial oligoelement preparation used:
Composition per vial | TE1 (10 ml) | TE4 (10 ml) |
Zinc | 38.2 µmol or 2.5 mg | 15.3 µmol or 1 mg |
Selenium | 0.253 µmol or 0.02 mg | 0.253 µmol or 0.02 mg |
Copper | 3.15 µmol or 0.2 mg | 3.15 µmol or 0.2 mg |
Iodine | 0.0788 µmol or 0.01 mg | 0.079 µmol or 0.01 mg |
Fluorine | 30 µmol or 0.57 mg | - |
Manganese | 0.182 µmol or 0.01 mg | 0.091 µmol or 0.005 mg |
Table 6: Composition of the commercial vitamin preparations used:
Composition per vial | V1 | V2 |
Vitamin B1 | 2.5 mg | - |
Vitamin B2 | 3.6 mg | - |
Niacinamide | 40 mg | - |
Vitamin B6 | 4.0 mg | - |
Pantothenic acid | 15.0 mg | - |
Biotin | 60 µg | - |
Folic acid | 400 µg | - |
Vitamin B12 | 5.0 µg | - |
Vitamin C | 100 mg | - |
Vitamin A | - | 2300 UI |
Vitamin D | - | 400 UI |
Vitamin E | - | 7 UI |
Vitamin K | - | 200 µg |
To perform an addition:
- It must be carried out under aseptic conditions.
- Prepare the injection point of the bag.
- Puncture the injection point and inject the additives using an injection needle or a reconstitution device.
- Mix the contents of the bag and the additives.
Preparation of the perfusion:
- It must be carried out under aseptic conditions.
- Hang the bag.
- Remove the plastic protector from the administration outlet.
- Firmly insert the tip of the perfusion equipment into the administration outlet.
Administration of the perfusion:
- For single use only.
- Administer the product only after opening the non-permanent seals between the two or three chambers and mixing the contents of the two or three chambers.
- Make sure the final activated 3-chamber bag emulsion does not show signs of phase separation and the final 2-chamber bag solution does not show signs of particles.
- It is recommended to use immediately after breaking the non-permanent seals. Do not store for later perfusion.
- Do not connect a partially used bag.
- The use of a 1.2 micras filter is recommended for the administration of Numeta G13%E.
- Do not connect bags in series to avoid a gas embolism due to the residual air contained in the main bag.
- When used in newborns and children under 2 years, it should be protected from light exposure until administration is completed.
The active ingredients are:
Active ingredient | B2C activated (240 ml) | B3C activated (300 ml) |
Amino acid compartment | ||
Alanine | 0.75 g | 0.75 g |
Arginine | 0.78 g | 0.78 g |
Aspartic acid | 0.56 g | 0.56 g |
Cysteine | 0.18 g | 0.18 g |
Glutamic acid | 0.93 g | 0.93 g |
Glycine | 0.37 g | 0.37 g |
Histidine | 0.35 g | 0.35 g |
Isoleucine | 0.62 g | 0.62 g |
Leucine | 0.93 g | 0.93 g |
Lysine monohydrate (equivalent to Lysine) | 1.15 g (1.03 g) | 1.15 g (1.03 g) |
Methionine | 0.22 g | 0.22 g |
Ornithine hydrochloride (equivalent to Ornithine) | 0.30 g (0.23 g) | 0.30 g (0.23 g) |
Phenylalanine | 0.39 g | 0.39 g |
Proline | 0.28 g | 0.28 g |
Serine | 0.37 g | 0.37 g |
Taurine | 0.06 g | 0.06 g |
Threonine | 0.35 g | 0.35 g |
Tryptophan | 0.19 g | 0.19 g |
Tyrosine | 0.07 g | 0.07 g |
Valine | 0.71 g | 0.71 g |
Potassium acetate | 0.61 g | 0.61 g |
Calcium chloride dihydrate | 0.55 g | 0.55 g |
Magnesium acetate tetrahydrate | 0.10 g | 0.10 g |
Sodium glycerophosphate hydrate | 0.98 g | 0.98 g |
Glucose compartment | ||
Glucose monohydrate (equivalent to anhydrous glucose) | 44.00 g (40.00 g) | 44.00 g (40.00 g) |
Lipid compartment | ||
Refined olive oil (approx. 80%) + refined soybean oil (approx. 20%) | - | 7.5 g |
B2C = 2-chamber bag, B3C = 3-chamber bag
The reconstituted solution/emulsion provides the following:
Composition | ||||
B2C activated | B3C activated | |||
Per unit of volume (ml) | 240 | 100 | 300 | 100 |
Nitrogen (g) | 1.4 | 0.59 | 1.4 | 0.47 |
Amino acids (g) | 9.4 | 3.9 | 9.4 | 3.1 |
Glucose (g) | 40.0 | 16.7 | 40.0 | 13.3 |
Lipids (g) | 0 | 0 | 7.5 | 2.5 |
Energy | ||||
Total calories (kcal) | 198 | 82 | 273 | 91 |
Non-protein calories (kcal) | 160 | 67 | 235 | 78 |
Glucose calories (kcal) | 160 | 67 | 160 | 53 |
Lipid calories (kcal) | 0 | 0 | 75 | 25 |
Non-protein calories/nitrogen (kcal/g N) | 113 | 113 | 165 | 165 |
Lipid calories (% non-protein calories) | N/A | N/A | 32 | 32 |
Lipid calories (% total calories) | N/A | N/A | 28 | 28 |
Electrolytes | ||||
Sodium (mmol) | 6.4 | 2.7 | 6.6 | 2.2 |
Potassium (mmol) | 6.2 | 2.6 | 6.2 | 2.1 |
Magnesium (mmol) | 0.47 | 0.20 | 0.47 | 0.16 |
Calcium (mmol) | 3.8 | 1.6 | 3.8 | 1.3 |
Phosphate (mmol) | 3.2 | 1.3 | 3.8 | 1.3 |
Acetate (mmol) | 7.2 | 3.0 | 7.2 | 2.4 |
Malate (mmol) | 3.2 | 1.3 | 3.2 | 1.1 |
Chloride (mmol) | 9.3 | 3.9 | 9.3 | 3.1 |
pH (approx.) | 5.5 | 5.5 | 5.5 | 5.5 |
Osmolality approx. (mOsm/L) | 1400 | 1400 | 1150 | 1150 |
(a) Includes calories from egg phospholipids for perfusion.
(b) Includes phosphate provided by egg phospholipids for perfusion.
The other components are:
L-Malic acid |
Hydrochloric acid |
Egg phospholipids for perfusion |
Glycerol |
Sodium oleate |
Sodium hydroxide |
Water for injectable preparations |
(a) for pH adjustment
Baxter and Numeta are registered trademarks of Baxter International Inc.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NUMETA G13% Emulsion for InfusionDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for NUMETA G13% Emulsion for Infusion
Discuss questions about NUMETA G13% Emulsion for Infusion, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















