
NOVOTHIRTEEN 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use NOVOTHIRTEEN 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NovoThirteen 2,500UI powder and solvent for solution for injection
catridecacog (recombinant coagulation factor XIII)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is NovoThirteen and what is it used for
- What you need to know before you use NovoThirteen
- How to use NovoThirteen
- Possible side effects
- Storage of NovoThirteen
- Contents of the pack and other information
1. What is NovoThirteen and what is it used for
What is NovoThirteen
NovoThirteen contains the active substance catridecacog, which is identical to human coagulation factor XIII, an enzyme necessary for blood coagulation. NovoThirteen replaces the missing factor XIII and promotes the stabilization of the initial blood clot by creating a mesh around it.
What is NovoThirteen used for
NovoThirteen is used to prevent bleeding in patients who do not have enough or are missing part of factor XIII (called subunit A).
2. What you need to know before you use NovoThirteen
It is important that you use NovoThirteen solution for injection immediately after preparation.
Do not use NovoThirteen
- If you are allergic to catridecacog or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, consult your doctor before using this medicine.
Warnings and precautions
Consult your doctor before starting treatment with NovoThirteen:
- If you have or have had a higher risk of blood clot formation (thrombosis), as NovoThirteen may increase the severity of a pre-existing blood clot.
- If you have or have had any liver injury.
Contact your doctor immediately:
- If you experience bleeding during treatment with NovoThirteen and it occurs spontaneously and/or requires treatment.
- If you experience an allergic reaction to NovoThirteen. Symptoms may include: hives, itching, swelling, difficulty breathing, low blood pressure (symptoms include paleness and cold skin, rapid pulse), dizziness, and sweating.
Other medicines and NovoThirteen
Tell your doctor if you are using, have recently used, or might use any other medicines.
It is not recommended to use NovoThirteen in combination with recombinant coagulation factor VIIa (another blood coagulation factor).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
NovoThirteen contains sodium
This medicine contains less than 23 mg (1 mmol) of sodium per injection, so it is essentially "sodium-free".
3. How to use NovoThirteen
Your treatment with NovoThirteen should be started by a doctor with experience in the treatment of rare bleeding disorders.
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor again.
Before using NovoThirteen for injection, you need to reconstitute the product. See the instructions for use of NovoThirteen.
NovoThirteen is administered intravenously. The dose will depend on your body weight. The usual dose for preventing bleeding is 35 UI per kilogram of body weight. Injections are given once a month (every 28 ± 2 days).
If you experience bleeding, you should contact your doctor who will decide if you need an injection.
NovoThirteen should be injected at a rate not exceeding 2 ml/minute.
According to the concentration of the NovoThirteen solution, the dose volume for injection (in milliliters) can be calculated using the following formula:
Dose volume in milliliters = 0.042 x your body weight in kilograms.
You should only use the dose prescribed and calculated by your doctor using this formula, taking into account that the usual dose and concentration of NovoThirteen are different from those of other medicines containing factor XIII.
Your doctor may adjust the dose if necessary.
Use in small children
For children who weigh less than 24 kg, reconstituted NovoThirteen should be further diluted with 6 ml of 0.9% sodium chloride solution for injection to adjust the dose for small children. See the section "Instructions for use of NovoThirteen - Instructions on how to dilute reconstituted NovoThirteen" for more information.
The dose volume for reconstituted NovoThirteen diluted with 6 ml of 0.9% sodium chloride solution for injection can be calculated using the following formula:
Dose volume in milliliters = 0.117 x body weight in kilograms.
Use in children and adolescents (who weigh more than 24 kg)
NovoThirteen can be used in adolescents and children in the same way as in adults, both for the prevention of bleeding and if bleeding occurs.
If you use more NovoThirteen than you should
Information on overdose of NovoThirteen is limited. None of the reported cases have shown symptoms of illness. Contact your doctor if you have injected a larger amount of NovoThirteen than indicated.
If you forget to use NovoThirteen
Consult your doctor if you forget to take a NovoThirteen injection. Do not use a double dose to make up for forgotten doses.
If you stop using NovoThirteen
If you stop using NovoThirteen, you will no longer be protected against bleeding. Do not stop using NovoThirteen without consulting your doctor, who will explain the consequences of stopping treatment and offer you other options.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects include:
Common:(may affect up to 1 in 10 people):
- Headache (the most common side effect)
- Pain at the injection site
- Pain in legs and arms
- Increased amount of small protein fragments due to the dissolution of blood clots
- Decreased number of some types of white blood cells. This means that your body may be more prone to infections
- Development of antibodies against factor XIII. These antibodies do not affect the effect of the medicine.
Side effects in children:
The side effects seen in children are the same as those seen in adults, but side effects may be more frequent in children than in adults.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Vigilance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of NovoThirteen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store in the original package to protect from light.
Once prepared, NovoThirteen for injection should be used immediately.
The solution is clear and colorless. Do not use this medicine if you notice particles or a change in color when reconstituted.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of NovoThirteen
- The active substance is catridecacog (recombinant coagulation factor XIII): 2,500 UI/3 ml, after reconstitution, corresponding to a concentration of 833 UI/ml.
- The other ingredients are, for the powder: sodium chloride, sucrose, and polysorbate 20, L-histidine, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), and for the solvent: water for injections.
Appearance of the product and pack contents
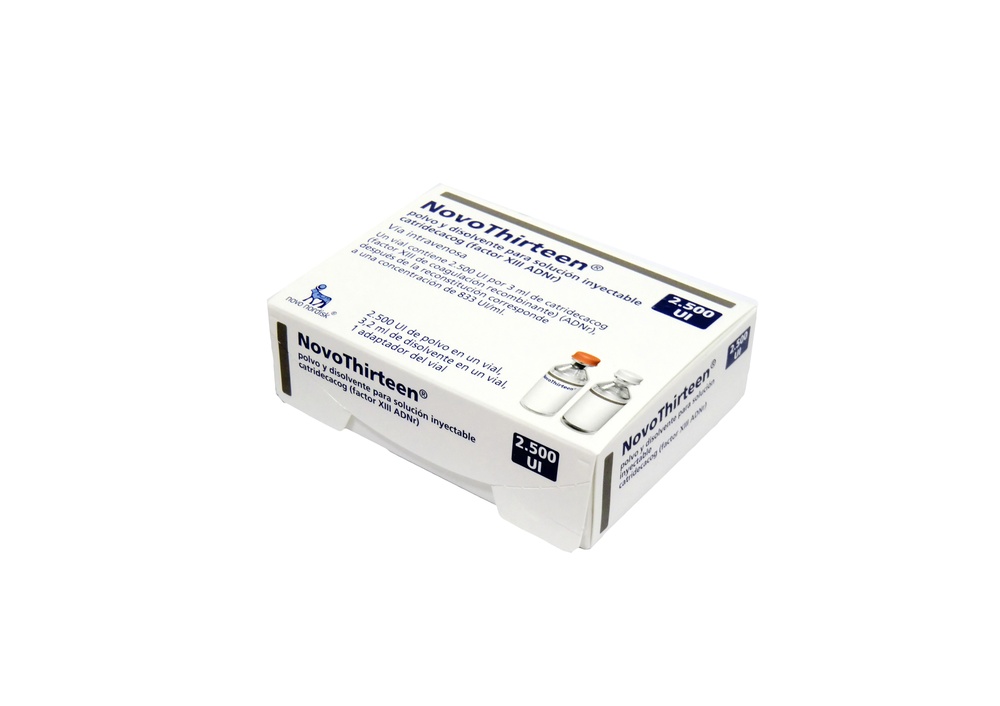
NovoThirteen is supplied as a powder and solvent for solution for injection (2,500 UI of powder in a vial and 3.2 ml of solvent in a vial, with a vial adapter).
Pack size of 1.
The powder is white and the solvent is clear and colorless.
Marketing authorisation holder and manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for use of NovoThirteen
To reconstitute and administer this medicine, you will need the following materials: a 10 ml syringe or a syringe of suitable size according to the injection volume, alcohol swabs, the vial adapter included, and an infusion set (tubing, butterfly needle).
Preparation of the solution
Always use aseptic technique. Before starting, wash your hands. Bring the powder and solvent vials to a temperature not above 25°C, keeping the vials in your hands until you feel they are at the same temperature as your hands. Remove the plastic caps from the 2 vials. Do not use the vials if the caps are loose or missing. Clean the rubber stoppers of the vials with an alcohol swab and let them dry before use.
The medicine is reconstituted using the included vial adapter.
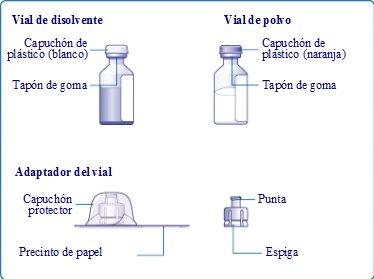
Remove the paper seal from the vial adapter without removing it from the protective cap. Place the vial adapter on the solvent vial (water for injections). Be careful not to touch the spike of the vial adapter.
Once attached, remove the protective cap from the vial adapter.
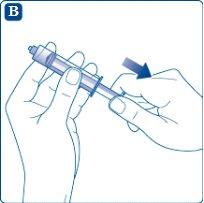
Pull the syringe plunger to draw up a volume of air equivalent to the total amount of solvent contained in the solvent vial.
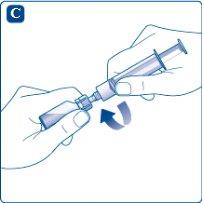
Screw the syringe firmly onto the vial adapter attached to the solvent vial. Inject air into the vial by pushing the plunger until you feel a clear resistance.
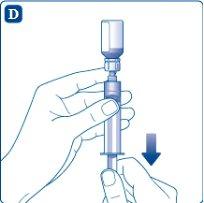
Hold the syringe with the solvent vial upside down. Pull the plunger to draw up the solvent and transfer it to the syringe.
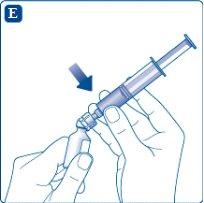
Remove the empty solvent vial by tilting the syringe attached to the vial adapter.
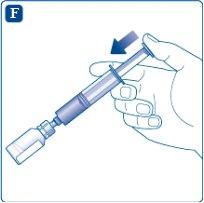
Adjust the vial adapter, still attached to the syringe, onto the powder vial until you hear a click. Hold the syringe slightly tilted with the vial downwards. Slowly push the plunger to inject the solvent into the powder vial. Make sure not to direct the jet of solvent directly onto the powder as this would create foam.
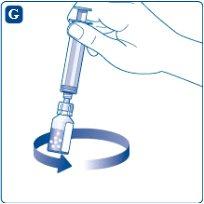
Make gentle circular movements with the vial until all the powder is dissolved. Do not shake the vial, as this would create foam.
NovoThirteen should be inspected visually for particulate matter and discoloration prior to administration. If either is observed, discard the medicine.
Reconstituted NovoThirteen is a clear and colorless solution.
If a larger dose is needed, repeat the procedure in a separate syringe until the required dose is reached.
Important information
Once prepared, NovoThirteen for injection should be used immediately.
If dilution of reconstituted NovoThirteen is needed, refer to the section "Dilution of reconstituted product with 0.9% sodium chloride solution for injection".
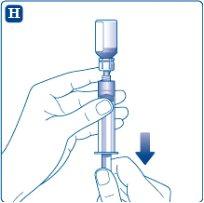
Make sure the plunger is pushed all the way down before turning the syringe (it may have been expelled by the pressure in the vial). Hold the syringe with the vial upside down and pull the plunger to draw up the calculated amount for the injection.
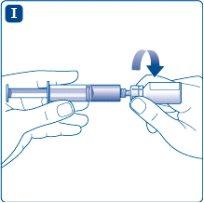
Unscrew the vial adapter with the vial.
The medicine is now ready to be injected into a vein. Follow the injection procedure as instructed by your doctor.
After injection
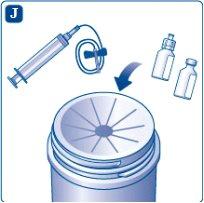
Discard the syringe, vial adapter, infusion set, and vials safely. Disposal of unused medicine and all materials that have come into contact with it should be done according to local regulations.
Instructions on how to dilute reconstituted NovoThirteen
To dilute reconstituted NovoThirteen, you will need the following materials: a vial containing 0.9% sodium chloride solution for injection, a 10 ml syringe, and alcohol swabs.
General instructions for dilution
Dilution should be carried out using aseptic techniques.
Carefully draw up exactly 6 ml of 0.9% sodium chloride solution for injection into the 10 ml syringe.
Slowly inject the 6 ml of 0.9% sodium chloride solution for injection into the vial of reconstituted NovoThirteen.
Make gentle circular movements with the vial to mix the solution.
The diluted solution is a clear and colorless solution. Check the solution for particles and discoloration. If you notice either, please discard it.
After dissolution, proceed to step H.
Any residual material from the diluted product should be discarded immediately.
If you have any doubts, ask your doctor or nurse.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NOVOTHIRTEEN 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for NOVOTHIRTEEN 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about NOVOTHIRTEEN 2500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















