
NICTUR 360 MICROGRAMS/ML ORAL SOLUTION


How to use NICTUR 360 MICROGRAMS/ML ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Nictur 360 micrograms/ml oral solution
(desmopressin)
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Nictur and what is it used for
- What you need to know before you take Nictur
- How to take Nictur
- Possible side effects
- Storing Nictur
Contents of the pack and other information
1. What is Nictur and what is it used for
The active substance that makes Nictur work (the active ingredient) is desmopressin. Desmopressin is very similar to a substance that is produced naturally in the body (the pituitary hormone vasopressin), which temporarily reduces the amount of urine produced by the body. This medicine is for oral use only.
This medicine is used to treat:
- Central diabetes insipidus, a disease that causes excessive thirst and the continuous production of large amounts of diluted urine as a result of insufficient production of the vasopressin hormone.
Bedwetting (involuntary urination during the night or primary nocturnal enuresis) in patients over 5 years of age with normal ability to concentrate urine.
2. What you need to know before you take Nictur
Do not take Nictur
- if you are allergic (hypersensitive) to desmopressin or any of the other ingredients of this medicine (listed in section 6),
- if you unusually drink large amounts of liquids (you have habitual or psychogenic polydipsia),
- if you take medicines that increase urine production (diuretics),
- if you have heart problems,
- if you have a predisposition or have low sodium levels in the blood (hyponatremia),
- if you have kidney problems,
- if you have uncontrolled high blood pressure,
- if you have the “Syndrome of Inadequate Secretion of the Antidiuretic Hormone” (SIADH).
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine.
During treatment with this medicine, avoid drinking excessive amounts of liquids because it can cause water retention in the body and/or low sodium levels in the blood with or without side effects (see section 4 Possible side effects).
Special care should be taken to avoid water retention in the body and low sodium levels in the blood in the following cases:
- if you are an elderly person,
- if you have a medical condition that causes fluid and/or electrolyte imbalancein the body, such as an infection, fever, or stomach upset.
- if you have severe bladder problems or alteration of urine flow,
- if you have asthma, epilepsy, and migraine,
- if you have kidney problems and/or cardiovascular diseases. In chronic kidney diseasesthe antidiuretic effect of desmopressin is less than usual.
Other medicines and Nictur
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, especially:
- tricyclic antidepressants or ISRS(used to treat depression),
- carbamazepine(used to treat epilepsy),
- chlorpromazine(used to treat psychosis or schizophrenia),
- medicines for pain and/or inflammationcalled non-steroidal anti-inflammatory drugs (NSAIDs), for example indomethacin, ibuprofen, acetylsalicylic acid,
- loperamide(used to treat diarrhea),
- diuretic agents.
These medicines increase the risk of fluid retention, which dilutes salt in the body.
- Dimethicone (used in the treatment of gas accumulation), due to decreased absorption of desmopressin.
Taking Nictur with food and drinks
At low doses, this medicine may be affected by food intake. If you notice that this medicine is less effective, you should take it without food before increasing the dose.
When using this medicine for bedwetting, reduce fluid intake to a minimum, from 1 hour beforetaking Nictur until 8 hours aftertaking a dose.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
It is preferable to avoid using this medicine during pregnancy.
Desmopressin passes into breast milk. If you are treated with desmopressin, you should stop breastfeeding.
Driving and using machines
Nictur does not affect the ability to drive or use machines.
Nictur contains methyl parahydroxybenzoate sodium and propyl parahydroxybenzoate sodium
This medicine may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate sodium and propyl parahydroxybenzoate sodium.
Nictur contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per ml; this is, essentially “sodium-free”.
3. How to take Nictur
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Usual dose
Treatment of central diabetes insipidus
Adults and children:Your doctor will adjust the dose individually. The initial doserecommended is 0.25 ml (90 micrograms) three times a day. Subsequently, the doctor will adjust the dose according to the response of each patient. The usual daily doseranges from 0.5 ml (180 micrograms) to 3 ml (1080 micrograms) of this medicine. The maintenance doseusually ranges from 0.25 ml to 0.5 ml (90 – 180 micrograms) of this medicine three times a day.
It is important to observe if symptoms of water retention in the body and/or low sodium levels in the blood appear (see section 4 Possible side effects). In this case, treatment will be interrupted and the dose will be adjusted again.
Bedwetting (involuntary urination during the night or primary nocturnal enuresis) in patients over 5 years of age:
Adults and children:the usual initial dose is 0.5 ml (180 micrograms) of this medicine one hour before bedtime. If this is not sufficiently active, the dose of this medicine can be increased to 1 ml (360 micrograms). The need to continue treatment is usually checked every three months, alternating a period without treatment for at least one week.
Elderly people:if the doctor decides to treat you, sodium levels in the blood should be measured before and three days after starting treatment and if the dose is increased or at any time your doctor considers it appropriate.
It is important to control fluid intake. If symptoms of water retention in the body and/or low sodium levels in the blood appear (see section 4 Possible side effects), treatment will be interrupted. Once treatment is restarted, fluid intake will be strictly controlled.
Instructions for use:
- Open the bottle (on the first opening the seal is broken).
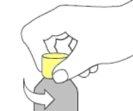
- Insert the oral syringe into the adapter and turn the bottle upside down to fill it with the dose to be administered.
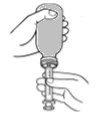
- Remove the oral syringe from the bottle and check that the correct amount is inside the syringe.
- Hold the syringe in the mouth and release the dose into the mouth.
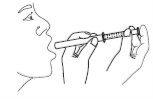
- Rinse with water after each use and close the bottle. Keep the bottle in the original packaging to protect it from light.
If you take more Nictur than you should
In case of overdose or accidental ingestion, contact your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 915 620 420, indicating the medicine and the amount ingested.
An overdose can prolong the effect of desmopressin and increase the risk of water retention in the body and/or low sodium levels in the blood. Symptoms may include headache, nausea, vomiting, weight gain, and in severe cases, convulsions. It is recommended to interrupt treatment, restrict fluid intake, and provide symptomatic treatment if necessary.
If you forget to take Nictur
Do not take a double dose to make up for forgotten doses.
If you stop taking Nictur
Do not stop taking Nictur before completing the treatment, as it may not have the expected effect. You should only change or stop the treatment if your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Nictur and go to see a doctor or the nearest hospital if the following occurs:
- Frequent(may affect up to 1 in 10 people): symptoms due to water retentionin the body, such as unusual or prolonged headache, feeling or being sick, unexplained weight gain, and in severe cases, convulsions, loss of consciousness.
- Very rare(may affect up to 1 in 10,000 people): allergic reactionssuch as rash, itching, fever, swelling of the lips, face, throat, or tongue causing difficulty swallowing or breathing.
Tell your doctor or pharmacist if you notice any of the following side effects:
- Very frequent(may affect more than 1 in 10 people): headache
- Frequent(may affect up to 1 in 10 people): abdominal pain, nausea.
- Very rare(may affect up to 1 in 10,000 people): emotional disorders in children, allergic reactions.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Nictur
Keep this medicine out of the sight and reach of children.
Do not store above 30°C. Store in the original packaging.
After the first opening, the medicine should be stored below 25°C for up to 8 weeks.
Do not use this medicine after the expiry date which is stated on the label and on the carton after EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Nictur
- The active substance is desmopressin. Each ml of oral solution contains 360 micrograms of desmopressin (as desmopressin acetate).
- The other ingredients are:
- Methyl parahydroxybenzoate sodium (E-219)
- Propyl parahydroxybenzoate sodium (E-217)
- Hydrochloric acid (for pH adjustment)
- Purified water.
Appearance of Nictur and contents of the pack
Nictur is a clear solution introduced into an amber glass bottle with a low-density polyethylene (LDPE) adapter, provided with a high-density polyethylene (HDPE) cap. The bottle contains 15 ml of solution. Each pack includes a 1.5 ml graduated syringe. The syringe is graduated from 0 to 1.5 ml, with divisions every 0.1 ml. The graduations corresponding to the doses of 0.25 ml, 0.5 ml, and 1.0 ml are specifically marked.
Nictur is available in individual packs (one 15 ml solution bottle and one graduated syringe) and in multipacks of 3 individual packs, each containing 15 ml of solution.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
GP-Pharm, S.A.
Polígono Industrial Els Vinyets – Els Fogars, sector 2
Carretera Comarcal C244, Km 22,
08777 – Sant Quintí de Mediona (Barcelona) SPAIN
Manufacturer
Laboratorio Reig Jofré S.A.
Gran Capità 10
08970 Sant Joan Despí – Barcelona
Spain
Elara Pharmaservices Europe Limited
239 Blanchardstown Corporate Park
Ballycoolin
Dublin D15 KV21
Ireland
This medicine is authorised in the Member States of the European Economic Area under the following names:
Spain: Nictur 360 micrograms/ml oral solution
Portugal: Nictur 0.36 mg/ml oral solution
Greece: Nictur 360 μικρογραμμ?ρια/ml π?σιμο δι?λυμα
United Kingdom: Desmopressin 360 micrograms/ml oral solution
Germany: Niwinas 360 Mikrogramm/ml Lösung zum Einnehmen
Date of last revision of this leaflet: January 2021
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NICTUR 360 MICROGRAMS/ML ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 360mcg/ml desmopressin (anhydrous base)Active substance: desmopressinManufacturer: Laboratorio Reig Jofre, S.A.Prescription requiredDosage form: SUBLINGUAL TABLET, 120 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: SUBLINGUAL TABLET, 240 microgramsActive substance: desmopressinManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for NICTUR 360 MICROGRAMS/ML ORAL SOLUTION
Discuss questions about NICTUR 360 MICROGRAMS/ML ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















