NGENLA 24 mg Injectable Solution in a Pre-filled Pen

How to use NGENLA 24 mg Injectable Solution in a Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ngenla 24mg solution for injection in pre-filled pen
somatropin
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you or the child under your care only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours or the child's under your care.
- If you or the child under your care get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ngenla and what is it used for
- What you need to know before you use Ngenla
- How to use Ngenla
- Possible side effects
- Storage of Ngenla
- Contents of the pack and other information
1. What is Ngenla and what is it used for
Ngenla contains the active substance somatropin, a modified pharmaceutical form of human growth hormone. Natural human growth hormone is necessary for bones and muscles to grow. It also helps fat and muscle tissues to develop in the right amounts. Ngenla is used to treat children and adolescents from 3 years of age who do not have enough growth hormone and are not growing at a normal rate.
The active substance of Ngenla is produced using "recombinant DNA technology". This means that it is created in cells that have been modified in the laboratory to produce it.
2. What you need to know before you use Ngenla
Do not use Ngenla
- If you or the child under your care are allergic to somatropin (see "Warnings and precautions") or to any of the other ingredients of this medicine (listed in section 6).
- If you or the child under your care have an active tumor (cancer). Tell your doctor if you or the child under your care have or have had an active tumor. Tumors must be inactive and you or the child under your care must have finished anti-tumor treatment before starting treatment with Ngenla.
- If you or the child under your care have stopped growing due to the closure of the growth plates (epiphyseal closure), which means that your doctor has told you or the child under your care that their bones have stopped growing.
- If you or the child under your care are severely ill (for example, suffering from complications after open-heart surgery, abdominal surgery, acute respiratory failure, multiple accidental injuries, or similar conditions). If you or the child under your care are about to undergo or have undergone major surgery, or are going to the hospital for any reason, inform your doctor and remind other doctors treating you or the child under your care that you or the child under your care are receiving growth hormone.
Warnings and precautions
Tell your doctor, pharmacist, or nurse before starting treatment with Ngenla:
- If you or the child under your care have a severe allergic reaction, stop using Ngenla and consult your doctor immediately. Sometimes, severe allergic reactions have occurred, such as hypersensitivity, including anaphylaxis or angioedema (difficulty breathing or swallowing, or swelling of the face, lips, throat, or tongue). If you or the child under your care have any of the following symptoms of a severe allergic reaction:
- Breathing problems.
- Swelling of the face, mouth, and tongue.
- Hives (urticaria, bumps that rise under the skin).
- Rash.
- Fever.
- If you or the child under your care are receiving replacement treatment with corticosteroids (glucocorticoids), you should consult your doctor regularly, as you or the child under your care may need an adjustment of your glucocorticoid dose.
- Your doctor should check from time to time if the thyroid gland is working properly in you or the child under your care and, if necessary, may prescribe treatment or adjust the dose of existing treatment, as this may be necessary for Ngenla to work properly.
- If you or the child under your care have Prader-Willi syndrome, you or the child under your care should not be treated with Ngenla unless you or the child under your care have a growth hormone deficiency.
- Your doctor should monitor the appearance in you or the child under your care of high blood sugar levels (hyperglycemia) during treatment with Ngenla. If you or the child under your care are receiving treatment with insulin or other diabetes medications, your doctor may need to adjust the insulin dose. If you or the child under your care have diabetes and a severe eye condition or one that is getting worse, you or the child under your care should not receive treatment with Ngenla.
- If you or the child under your care have ever had any type of tumor (cancer).
- If you or the child under your care experience changes in vision, severe or frequent headaches, related to nausea, vomiting, or experience loss of muscle control or coordination of voluntary movements, such as walking or grasping objects, difficulty speaking, eye movement, or difficulty swallowing, especially at the start of treatment, inform your doctor immediately. These could be signs of a temporary increase in pressure inside the brain (intracranial hypertension).
- If you or the child under your care are severely ill (for example, suffering from complications after open-heart surgery, abdominal surgery, acute respiratory failure, multiple accidental injuries, or similar conditions). If you or the child under your care are about to undergo or have undergone major surgery, or are going to the hospital for any reason, inform your doctor and remind other doctors treating you or the child under your care that you or the child under your care are receiving growth hormone.
- If you or the child under your care experience severe stomach pain during treatment with Ngenla, as it could be a symptom of pancreatitis.
- If you or the child under your care notice a lateral curvature of the spine (scoliosis), you or the child under your care should be examined frequently by your doctor.
- If during growth, you or the child under your care experience limping or pain in the hip or knee, you or the child under your care should consult your doctor immediately. These could be symptoms of bone disorders in the hip, as this can occur during periods of rapid growth.
- If you or the child under your care are taking or stopping oral contraceptives or hormone replacement therapy with estrogens, your doctor may recommend adjusting the dose of Ngenla.
Other medicines and Ngenla
Tell your doctor, pharmacist, or nurse if you or the child under your care are using, have recently used, or might use any other medicines.
- If you or the child under your care are receiving replacement treatment with corticosteroids (glucocorticoids), as these may reduce the effect of Ngenla on growth. You or the child under your care should consult your doctor regularly, as you or the child under your care may need an adjustment of your glucocorticoid dose.
- If you or the child under your care are receiving treatment with insulin or other diabetes medications, you should consult your doctor, as it may be necessary to adjust the dose.
- If you or the child under your care are receiving thyroid hormone treatment, your doctor may need to adjust the dose.
- If you or the child under your care are receiving oral estrogens, you should consult your doctor, as you or the child under your care may need to adjust the dose of Ngenla.
- If you or the child under your care are receiving cyclosporin (a medicine that weakens the immune system after a transplant), you should consult your doctor, as your doctor may need to adjust the dose.
- If you or the child under your care are receiving medicines to control epilepsy (anticonvulsants), you should consult your doctor, as your doctor may need to adjust the dose.
Pregnancy and breastfeeding
If you or the girl under your care are pregnant or breastfeeding, think you or the girl under your care may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Ngenla has not been tested in pregnant women and it is not known whether this medicine can harm the fetus. Therefore, it is preferable to avoid Ngenla during pregnancy. If you can become pregnant, you should not use Ngenla unless you are also using a reliable contraceptive method.
It is not known whether somatropin can pass into breast milk. Inform your doctor or the doctor of the girl under your care if you or the girl under your care are breastfeeding or plan to breastfeed. Your doctor will help you decide whether you or the girl under your care should stop breastfeeding or stop receiving Ngenla, taking into account the benefit of breastfeeding for the baby and the benefit of Ngenla for you or the girl under your care.
Driving and using machines
Ngenla does not affect the ability to drive and use machines.
Ngenla contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially "sodium-free".
Ngenla contains metacresol
Ngenla contains a preservative called metacresol. In very rare cases, the presence of metacresol may cause inflammation (swelling) in the muscles. If you or the child under your care experience muscle pain or pain at the injection site, inform your doctor.
3. How to use Ngenla
This medicine will only be prescribed by a doctor with experience in growth hormone treatment and who has confirmed your diagnosis or that of the child under your care.
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are in doubt, consult your doctor, pharmacist, or nurse again.
Your doctor will decide the dose of Ngenla to be injected.
How much to use
Your doctor will calculate your dose of Ngenla based on your body weight in kilograms. The recommended dose is 0.66 mg per kg of body weight and is administered once a week. If you or the child under your care have been previously treated with daily growth hormone injections, your doctor will tell you to wait before receiving the first dose of Ngenla until the day after your last daily injection and then continue with Ngenla once a week.
Do not change your dose unless your doctor tells you to.
How Ngenla is administered
- Ngenla is available as a pre-filled pen in 2 different sizes (Ngenla 24 mg and Ngenla 60 mg). Depending on the recommended dose, your doctor or the doctor of the child under your care will prescribe the most suitable pen size (see section 6 "Contents of the pack and other information").
- Before you or the child under your care use the pen for the first time, your doctor or nurse will teach you how to use it. Ngenla is administered by injection under the skin (subcutaneous injection) using a pre-filled pen. Do not inject it into a vein or muscle.
- The best area for administering Ngenla is the abdomen (stomach), thighs, buttocks, and upper arms. Injections in the upper arms and buttocks should be administered by the caregiver.
- Change the injection site on your body or the body of the child under your care each time a dose is administered.
- If more than one injection is required to administer a complete dose, each should be administered in a different injection site.
Detailed instructions for use of the pre-filled pen are found at the end of this leaflet.
When to use Ngenla
You or the child under your care should use this medicine once a week on the same day each week.
You or the child under your care should record which day of the week you use Ngenla to help you remember to inject this medicine once a week.
If necessary, you or the child under your care can change the day of your weekly injection as long as at least 3 days have passed since you or the child under your care received the last injection. After selecting a new day of administration, continue administering the injection to yourself or the child under your care on that day each week.
If you use more Ngenla than you should
If you or the child under your care have injected more Ngenla than you should, contact your doctor immediately, as it may be necessary to monitor your blood sugar levels.
If you forget to use Ngenla
If you or the child under your care forgot to inject a dose and:
- It has been 3 days or less since you or the child under your care should have used Ngenla, use it as soon as you remember. Then, inject your next dose on your usual injection day.
- It has been more than 3 days since you or the child under your care should have used Ngenla, skip the missed dose. Then, inject your next dose as usual on the next scheduled day. You should maintain a regular injection day.
Do not use a double dose to make up for the missed dose.
If you stop treatment with Ngenla
Do not stop using this medicine without consulting your doctor.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common: may affect more than 1 in 10 people
- Headache
- Bleeding, inflammation, itching, pain, redness, discomfort, stinging, sensitivity, or warmth at the injection site (injection site reactions)
- Fever (pyrexia)
Common: may affect up to 1 in 10 people
- Decrease in the number of red blood cells in the blood (anemia)
- Increase in the number of eosinophils in the blood (eosinophilia)
- Decrease in thyroid hormone levels in the blood (hypothyroidism)
- Allergic inflammation of the conjunctiva, the transparent layer that covers the outside of the eye (allergic conjunctivitis)
- Joint pain (arthralgia)
- Pain in arms or legs
Uncommon: may affect up to 1 in 100 people
- The adrenal glands do not produce enough steroid hormones (adrenal insufficiency)
- Rash
Other possible side effectsnot observed withNgenlabut which have beenreportedwith other medicinal growth hormone treatments may include the following:
- Growth of tissues (non-cancerous or cancerous)
- Type 2 diabetes
- Increased intracranial pressure (which causes symptoms such as severe headache, visual disturbances, or vomiting)
- Numbness or tingling
- Pain in the joints or muscles
- Enlargement of the breasts in children and men
- Skin rash, redness, and itching
- Water retention (which manifests as swollen fingers or ankles)
- Swelling of the face
- Pancreatitis (which causes symptoms of stomach pain, nausea, vomiting, or diarrhea)
In very rare cases, the presence of metacresol may cause inflammation (swelling) in the muscles. If you or the child under your care experience muscle pain or pain at the injection site, inform your doctor.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ngenla
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pen or on the carton after “EXP”. The expiry date is the last day of the month indicated.
The pre-filled pen should not be used for more than 28 days after the first use.
Before the first use of Ngenla
- Store in a refrigerator (between 2 °C and 8 °C).
- Keep Ngenla in the outer packaging to protect it from light.
- Remove Ngenla from the refrigerator before use. Ngenla can be kept at room temperature (up to a maximum of 32 °C) for up to 4 hours.
- Do not use this medicine if you notice that the solution is cloudy or dark yellow in color. Do not use the medicine if it has scales or particles.
- Do not shake the pen. Shaking it can damage the medication.
After the first use of Ngenla
- Use it within 28 days after the first use. Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
- Keep Ngenla with the pen cap on to protect it from light.
- Do not store the pre-filled pen with the needle attached.
- Discard the pen after the last dose, even if it contains unused medicine.
- Ngenla can be kept at room temperature (up to a maximum of 32 °C) for up to 4 hours with each injection, up to a maximum of 5 times. Return Ngenla to the refrigerator after each use.
- Do not leave it at room temperature for more than 4 hours with each use.
- Do not place the pen in any location where the temperature exceeds 32 °C.
- If more than 28 days have passed since the first use of the pen, dispose of it even if it contains unused medicine. If your pen or the pen of the child under your care has been exposed to temperatures above 32 °C, has been removed from the refrigerator for more than 4 hours with each use, or has been used a total of 5 times, dispose of it even if it contains unused medicine.
To help you remember when to discard your pen, you can write the date of the first use on the pen label.
A small amount of medicine may remain in the pen after all doses have been administered correctly. Do not attempt to use the remaining medicine. After administering the last dose, the pen should be disposed of properly.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Ngenla Composition
- The active ingredient is somatropin.
Ngenla 24 mg injectable solution in a pre-filled pen
One ml of solution contains 20 mg of somatropin.
Each pre-filled pen contains 24 mg of somatropin in 1.2 ml of solution. Each pre-filled pen provides doses of 0.2 mg to 12 mg in a single injection in increments of 0.2 mg.
Ngenla 60 mg injectable solution in a pre-filled pen
One ml of solution contains 50 mg of somatropin.
Each pre-filled pen contains 60 mg of somatropin in 1.2 ml of solution. Each pre-filled pen provides doses of 0.5 mg to 30 mg in a single injection in increments of 0.5 mg.
- The other ingredients are: trisodium citrate dihydrate, citric acid monohydrate, L-histidine, sodium chloride (see section 2 "Ngenla contains sodium"), poloxamer 188, m-Cresol, and water for injectable preparations.
Product Appearance and Container Contents
Ngenla is a clear and colorless to slightly yellowish injectable solution in a pre-filled pen.
Ngenla 24 mg injectable solution is available in a pack size containing 1 pre-filled pen. The pen cap, dose button, and pen label are purple.
Ngenla 60 mg injectable solution is available in a pack size containing 1 pre-filled pen. The pen cap, dose button, and pen label are blue.
Marketing Authorization Holder Pfizer Europe MA EEIG Boulevard de la Plaine 17 1050 Bruxelles Belgium |
Manufacturer Pfizer Manufacturing Belgium NV Rijksweg 12 2870 Puurs-Sint-Amands Belgium |
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
България Пфайзер България ЕООД Тел.: +359 2 970 4333 | Magyarország Pfizer Kft. Tel.: + 36 1 488 37 00 |
Česká republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf.: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλάδα Pfizer Ελλάς Α.Ε. Τηλ: +30 210 6785800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: + 386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κύπρος Pfizer Ελλάς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 |
Date of Last Revision of this Leaflet 04/2025.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency web site: https://www.ema.europa.eu.
Instructions for Use
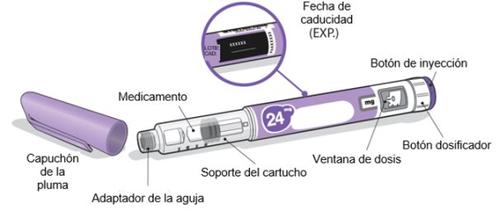
Ngenla 24 mg Pen
Injection for subcutaneous use only
Keep this leaflet. These instructions show you step by step
how to prepare and administer an injection of Ngenla.
Important Information about the Ngenla Pen
- Ngenla injectable is a pre-filled, multi-dose pen that contains 24 mg of medication.
- Ngenla injectable can be administered by a patient, caregiver, doctor, nurse, or pharmacist. Do notattempt to inject Ngenla yourself until you have been shown the correct way to administer the injections and have read and understood the Instructions for Use. If your doctor, nurse, or pharmacist decides that you or a caregiver can administer the Ngenla injections at home, you should receive training on the correct way to prepare and inject Ngenla. It is important that you consult with your doctor, nurse, or pharmacist to ensure that you understand the administration instructions for Ngenla.
- To help you remember when to inject Ngenla, you may mark your calendar in advance. Call your doctor, nurse, or pharmacist if you or your caregiver have any questions about the correct way to inject Ngenla.
- Each turn (click) of the dose button increases the dose by 0.2 mg of medication. You can administer from 0.2 mg to 12 mg in a single injection. If your dose is more than 12 mg, you will need to administer more than 1 injection.
- A small amount of medication may remain in the pen after all doses have been administered correctly. This is normal. Patients should not attempt to use the remaining solution, but should dispose of the pen properly.
- Do notshare your pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
- Always use a new, sterile needle for each injection. This will reduce the risk of contamination, infection, medication loss, and needle blockage that can lead to an incorrect dose.
- Do notshake the pen. Shaking the pen can damage the medication.
- It is not recommendedto use the pen by blind or visually impaired persons without the assistance of a person trained in the proper use of the product.
Materials You Will Need Each Time You Inject
Supplied with the Pen:
- 1 Ngenla 24 mg pen.
Not Supplied with the Pen:
- 1 new, sterile needle for each injection.
- Alcohol swabs.
- Cotton balls or gauze pads.
- Adhesive bandage.
- A suitable sharps container for disposal of the pen needle and pen.
Ngenla 24 mg Pen:
Needles That Can Be Used
The needles for the pen are not includedwith the Ngenla pen. You can use needles from 4 mm to 8 mm and between 30G and 32G.
- The following needles have been shown to be compatible with the Ngenla pen:
- 32G (Novo Nordisk, NovoFine Plus)
- 31G (Novo Nordisk, NovoFine)
- 31G (Becton Dickinson and Company, BD Ultra-Fine or BD Micro-Fine)
- The following safety needles have been shown to be compatible with the Ngenla pen:
- 30G (Becton Dickinson and Company, AutoShield Duo)
- 30G (Novo Nordisk, NovoFine AutoCover)
- Consult your doctor, nurse, or pharmacist about the suitable needle for you.
Sterile Needle (Example) Not Included:
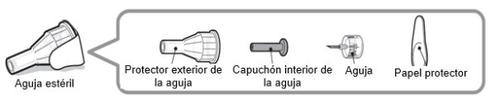
Note:Safety needles do not have an inner needle cap. Steps 5, 6, and 11 of these instructions related to the inner needle cap may not be applicable when using a safety needle. Consult the manufacturer's instructions for use of the needle for more information.
Caution:Never use a bent or damaged needle. Always handle pen needles with care to avoid accidentally pricking yourself (or others) with the needle. Do notput a new needle on the pen until you are ready for the injection.
Preparing for Injection
Step 1 Preparation
- Wash and dry your hands.
- You can use your pen directly from the refrigerator. For a more comfortable injection, let your pen come to room temperature for up to 30 minutes. (See section 5 "Storage of Ngenla" of the Ngenla 24 mg pre-filled pen leaflet).
- Check the name, concentration, and label of the pen to ensure it is the medication that your doctor has prescribed.
- Check the expiration date on the pen label. Do notuse it if it has passed the expiration date.
- Do notuse the pen if:
- It has been frozen or exposed to heat (above 32°C) or it has been more than 28 days since the first use of the pen. (See section 5 "Storage of Ngenla" of the Ngenla 24 mg pre-filled pen leaflet).
- It has been dropped.
- It appears to be broken or damaged.
- Do notremove the pen cap until you are ready for the injection.
Step 2 Choose and Clean the Injection Site
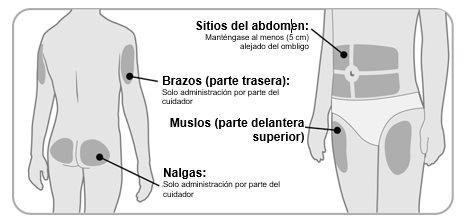
- Ngenla can be administered in the abdomen (stomach), thighs, buttocks, or the upper arms.
- Choose the best injection site, as recommended by your doctor, nurse, or pharmacist.
- If more than 1 injection is needed to complete your dose, each injection should be administered in a different injection site.
- Do notinject into areas of bone, bruised, red, sore, or hard areas, or areas with scars or skin conditions.
- Clean the injection site with an alcohol swab.
- Let the injection site dry.
- Do nottouch the injection site after cleaning.
Step 3 Check the Medication
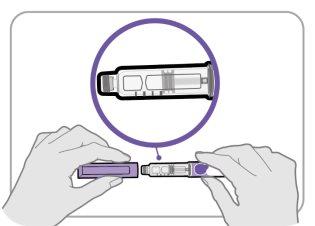
- Remove the pen cap and set it aside for later.
- Check the medication inside the cartridge holder.
- Make sure the medication is clear and colorless to slightly yellowish. Do notinject the medication if it is cloudy or dark yellow.
- Make sure the medication is free of flakes or particles. Do notinject the medication if it has flakes or particles.
Note:It is normal to see one or more air bubbles in the medication.
Step 4 Attach the Needle
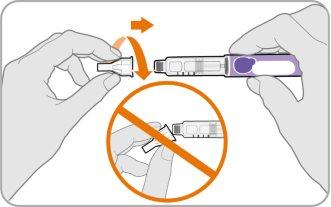
- Take a new needle and remove the paper protector.
- Align the needle with your pen, keeping them straight.
- Gently push and then screw the needle onto your pen.
Do notovertighten.
Note:Be careful not to put the needle on at an angle. This can cause the pen to leak.
Caution:Needles have sharp points on both ends. Be careful to avoid accidentally pricking yourself (or others) with the needle.
Step 5 Remove the Outer Needle Protector
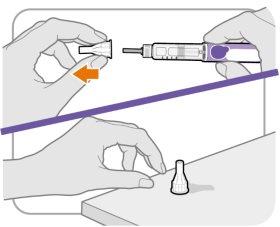
- Remove the outer needle protector.
- Make sure to keep the outer needle protector. You will need it later to remove the needle.
Note:You should see an inner needle cap after removing the outer needle protector. If you do not see this, try putting the needle on again.
Note:If you are using a safety needle, consult the manufacturer's instructions for use.
Step 6 Remove the Inner Needle Cap
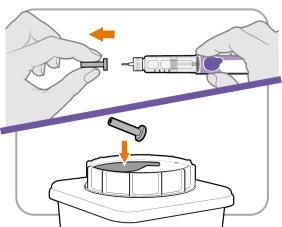
- Remove the inner needle cap carefully to expose the needle.
- Dispose of the inner needle cap in a sharps container. It is no longer needed.
Note:If you are using a safety needle, consult the manufacturer's instructions for use.
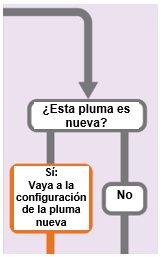
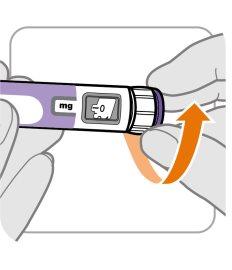
("Yes: Go to new pen setup" has an arrow indicating "New pen setup (priming)" and "No" has an arrow indicating "Set your prescribed dose")
New Pen Setup (Priming): Only for the First Use of a New Pen
You must set up each new pen (prime) before using it for the first time
- New pen setup is performed before each new pen is used for the first time.
- The purpose of setting up a new pen is to remove air bubbles and ensure that you receive the correct dose.
Important:Skip from Step A to Step C if you have already set up the pen.
Step A: Set the Dose to 0.4
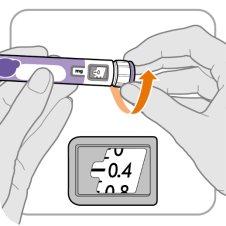
- Turn the dose button to 0.4.
Note:If you turn the dose button too far, you can go back.
Step B: Tap the Cartridge Holder
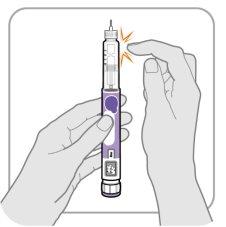
- Hold the pen with the needle pointing up so that air bubbles can rise.
- Gently tapthe cartridge holder to make the air bubbles float to the top.
Important:Follow Step B even if you do not see air bubbles.
Step C: Press the Button and Check for Liquid
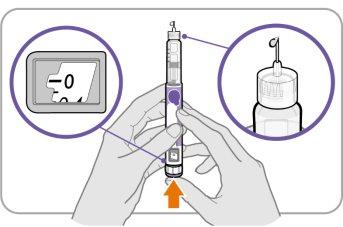
- Press the injection buttonuntil it cannot go further and "0"appears in the dose window.
- Checkif there is liquid at the tip of the needle. If liquid appears, your pen is set up.
- Always make sure that a drop of liquid appears before injection. If liquid does not appear, repeat from Step A to Step C.
- If liquid does not appear after repeating from Step A to Step C five (5) times, put on a new needle and try one (1) more time.
Do notuse the pen if a drop of liquid still does not appear. Contact your doctor, nurse, or pharmacist and use a new pen.
Set Your Prescribed Dose
Step 7 Set Your Dose
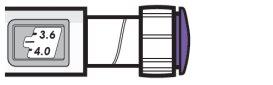
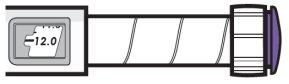
| Example A
3.8 mg is shown in the dose window Example B
12.0 mg is shown in the dose window |
- Turn the dose button to set your dose.
- The dose can be increased or decreased by turning the dose button in either direction.
- The dose button clicks 0.2 mg each time.
- Your pen contains 24 mg of medication, but you can only set a dose of up to 12 mg for a single injection.
- The dose window shows the dose in mg. See Examples A and B.
After having adjusted the correct dose.
Important:Donotpress the injection button while adjusting your dose.
What should I do if I cannot adjust the dose I need?
- If your dose is higher than 12 mg, you will need more than 1 injection.
- You can administer from 0.2 mg to 12 mg in a single injection.
- If you need help dividing your dose correctly, consult your doctor, nurse, or pharmacist.
- Use a new needle for each injection (see Step4: Place the needle).
- If you normally need to administer 2 injections for your full dose, make sure to administer your second dose.
What should I do if there is not enough medicine left in my pen?
- If your pen contains less than 12 mg of medicine, the dosing button will stop with the remaining amount of medicine shown in the dose window.
- If there is not enough medicine left in your pen for your full dose, you can:
- Inject the remaining amount in your pen and then prepare a new pen to complete your dose.
Remember to subtract the dose you have already received. For example, if the dose is 3.8 mg and you can only adjust the dosing button to 1.8 mg, you must inject another 2.0 mg with a new pen.
- Or get a new pen and inject the full dose.
Inject your dose
Step8 Insert the needle
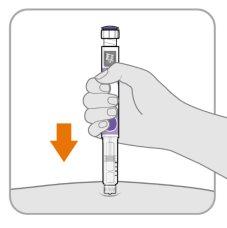
- Hold the pen so that you can see the numbers in the dose window.
- Insert the needle directly into the skin.
Step9 Inject the medicine
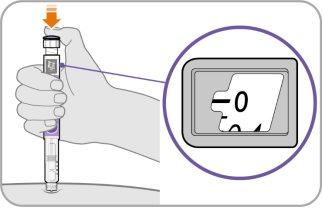
- Continue to hold the needle in the same position on your skin.
- Press the injection buttonuntil it cannot move further and “0”appears in the dose window.
Step10 Count to10
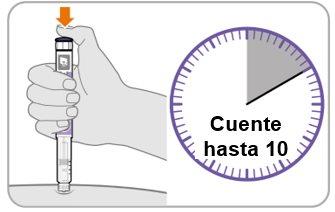
- Continue to press the injection button while counting to10. Counting to 10 will allow the full dose of medicine to be administered.
- After counting to 10, release the injection button and slowly remove the pen from the injection site by pulling the needle outwards.
Note:You may see a drop of medicine on the tip of the needle. This is normal and does not affect the dose you just received.
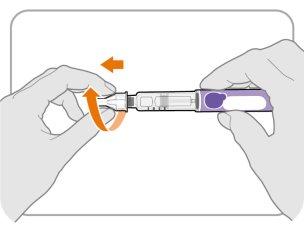
Step11 Place the outer needle shield
- Carefully replace the outer needle shield over the needle.
- Press the outer needle shield until it is secure.
Precaution:Never attempt to replace the inner needle cap on the needle. You may prick yourself with the needle.
Note:If you are using a safety needle, refer to the manufacturer's instructions for use.
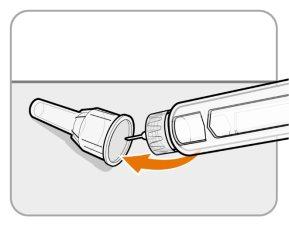
Step12 Remove the needle
- Unscrew the needle from the pen.
- Gently pull until the needle comes out.
Note:If the needle is still on, replace the outer needle shield and try again. Make sure to apply pressure when unscrewing the needle.
Discard used pen needles in a puncture-proof container as instructed by your doctor, nurse, or pharmacist and in accordance with local health and safety regulations. Keep the puncture-proof container out of the reach of children. Donotreuse needles.
Step13 Replace the pen cap
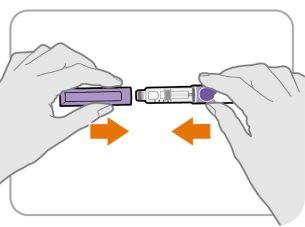
- Replace the pen cap on the pen.
- Donotcover the pen with a needle on.
- If there is any medicine left in your pen, store it in the refrigerator between uses (see Section5 “Storing Ngenla” of the Ngenla 24mg pre-filled pen leaflet).
Step14 After injection
- Press lightly on the injection site with a cotton ball or gauze pad and hold for a few seconds.
- Donotrub the injection site. You may have slight bleeding. This is normal.
- You can cover the injection site with a small adhesive bandage, if necessary.
- If the pen is empty or it has been more than 28daysafter the first use, discard it even if it contains unused medicine. Discard the pen in the puncture-proof container.
- To help you remember when to discard the pen, you can write the date of the first use on the pen label and the following:
Date of first use ______ / ______ / ______
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NGENLA 24 mg Injectable Solution in a Pre-filled PenDosage form: INJECTABLE, 60 mg / 1.2 mlActive substance: somatrogonManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for NGENLA 24 mg Injectable Solution in a Pre-filled Pen
Discuss questions about NGENLA 24 mg Injectable Solution in a Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions