
NEUPOGEN 48 MU (0.96 mg/ml) PRE-FILLED SYRINGE SOLUTION

How to use NEUPOGEN 48 MU (0.96 mg/ml) PRE-FILLED SYRINGE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Neupogen 48 MU (0.96 mg/ml)
Injectable Solution in Pre-filled Syringe
filgrastim
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Neupogen and what is it used for
- What you need to know before you use Neupogen
- How to use Neupogen
- Possible side effects
- Storage of Neupogen
- Contents of the pack and further information
- Instructions for injecting Neupogen
1. What is Neupogen and what is it used for
Neupogen is a white blood cell growth factor (granulocyte-colony stimulating factor) and belongs to a group of medicines called cytokines. Growth factors are proteins that are produced naturally in the body, but can also be produced using biotechnology for use as medicines. Neupogen works by making the bone marrow produce more white blood cells.
A decrease in the number of white blood cells (neutropenia) can occur for various reasons and makes your body less able to fight infections. Neupogen stimulates the bone marrow to produce new white blood cells quickly.
Neupogen can be used:
- to increase the number of white blood cells after chemotherapy to help prevent infections;
- to increase the number of white blood cells after a bone marrow transplant to help prevent infections;
- before receiving high-dose chemotherapy so that the bone marrow produces more stem cells that can be collected and given back to you after treatment. These cells can be collected from you or a donor. The stem cells will then return to the bone marrow and produce blood cells;
- to increase the number of white blood cells if you have severe chronic neutropenia to help prevent infections;
- in patients with advanced HIV infection to help reduce the risk of infections.
2. What you need to know before you use Neupogen
Do not use Neupogen
- if you are allergic to filgrastim or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Neupogen.
Tell your doctor before starting treatment if you have:
- sickle cell anaemia, as Neupogen may cause you to have a sickle cell crisis.
- a latex allergy. The needle cap on the pre-filled syringe contains a type of natural rubber called latex, which may cause an allergic reaction.
- osteoporosis (bone disease).
Tell your doctor immediately if during treatment with Neupogen:
- you have sudden signs of an allergic reaction, such as rash, itching, or hives on the skin, swelling of the face, lips, tongue, or other parts of the body, shortness of breath, wheezing, or difficulty breathing, which may be signs of a severe allergic reaction (hypersensitivity).
- you experience swelling of the face or ankles, blood in the urine, or brown colour in the urine, or if you notice that you are urinating less often than normal (glomerulonephritis).
- you have pain in the upper left side of your abdomen (stomach), pain in the lower left side of your ribcage, or pain in the left shoulder tip (these may be symptoms of an enlarged spleen (splenomegaly) or a possible rupture of the spleen).
- you experience bleeding or unusual bruising (these may be symptoms of a decrease in blood platelets (thrombocytopenia), with a reduced ability of the blood to clot).
- if you have symptoms of inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body), this has rarely been reported in patients with cancer and in healthy donors. Symptoms may include fever, abdominal pain, general malaise, back pain, and increased inflammatory markers. Tell your doctor if you experience these symptoms.
Loss of response to filgrastim
If you experience a loss of response or if you do not achieve a response to treatment with filgrastim, your doctor will investigate the causes, including whether you have developed antibodies that may neutralize the activity of filgrastim.
Your doctor may want to closely monitor you, see section 4 of the package leaflet.
If you are a patient with severe chronic neutropenia, you may be at risk of developing blood cancer (leukaemia, myelodysplastic syndrome (MDS)). You should discuss with your doctor the risk of developing blood cancer and what tests should be performed. If you develop blood cancer, you should not use Neupogen, unless your doctor advises you to.
If you are a stem cell donor, you must be between 16 and 60 years old.
Be careful with other products that stimulate white blood cells
Neupogen belongs to a group of products that stimulate the production of white blood cells. The healthcare professional treating you should always record the exact product you are using.
Other medicines and Neupogen
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breast-feeding
Neupogen has not been tested in pregnant or breast-feeding women.
Neupogen is not recommended during pregnancy.
It is important that you tell your doctor:
- if you are pregnant or breast-feeding;
- if you think you may be pregnant; or
- if you are planning to have a baby.
If you become pregnant during treatment with Neupogen, please tell your doctor.
Unless your doctor tells you otherwise, you must stop breast-feeding if you use Neupogen.
Driving and using machines
Neupogen may have a minor influence on your ability to drive and use machines. This medicine may cause dizziness. It is recommended that you wait and see how you react after administration of Neupogen before driving or using machines.
Neupogen contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per pre-filled syringe, which is essentially “sodium-free”.
Neupogen contains sorbitol
This medicine contains 50 mg of sorbitol in each ml.
Sorbitol is a source of fructose. If you (or your child) have hereditary fructose intolerance (HFI), a rare genetic disorder, you (or your child) should not receive this medicine. Patients with HFI cannot digest fructose, which may cause serious side effects.
You should consult your doctor before receiving this medicine if you (or your child) have HFI or if your child cannot eat sweet foods or drinks because they cause nausea, vomiting, or unpleasant side effects such as bloating, abdominal cramps, or diarrhoea.
3. How to use Neupogen
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor, nurse, or pharmacist again.
How is Neupogen administered and what dose should I use?
Neupogen is usually given as a daily injection under the skin (this is called a subcutaneous injection). It can also be given as a daily slow injection into a vein (this is called an intravenous infusion). The usual dose varies depending on your illness and weight. Your doctor will tell you how much Neupogen to use.
Patients undergoing bone marrow transplantation after chemotherapy:
Normally, you will receive your first dose of Neupogen at least 24 hours after chemotherapy and at least 24 hours after bone marrow transplantation.
You or the people caring for you can learn how to give subcutaneous injections so that you can continue your treatment at home. However, do not attempt to do this unless your healthcare professional has taught you how to do it correctly.
For how long will I need to use Neupogen?
You will need to use Neupogen until your white blood cell count is normal. You will have regular blood tests to check the number of white blood cells in your body. Your doctor will tell you for how long you will need to use Neupogen.
Use in children
Neupogen is used to treat children who are receiving chemotherapy or who have a very low white blood cell count (neutropenia). The dose for children receiving chemotherapy is the same as for adults.
If you use more Neupogen than you should
Do not increase the dose that your doctor has given you. If you think you have injected more Neupogen than you should, contact your doctor as soon as possible.
If you forget to use Neupogen
If you have missed an injection or have injected less than you should, contact your doctor as soon as possible. Do not take a double dose to make up for forgotten doses.
If you have any further questions on the use of this medicine, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediatelyduring treatment:
- if you have an allergic reaction that includes weakness, low blood pressure, difficulty breathing, swelling of the face, lips, tongue, or throat, and difficulty swallowing (anaphylaxis), rash, itching, or hives on the skin, swelling of the face, lips, tongue, or throat, and difficulty breathing (angioedema) and difficulty breathing (dyspnoea).
- if you have cough, fever, and difficulty breathing (dyspnoea), as these may be signs of acute respiratory distress syndrome (ARDS).
- if you have kidney damage (glomerulonephritis). Kidney damage has been observed in patients receiving Neupogen. Tell your doctor immediately if you experience swelling of the face or ankles, blood in the urine, or brown colour in the urine, or if you notice that you are urinating less often than normal.
- if you have any of the following side effects or a combination of them:
- swelling, which may be associated with urinating less often, difficulty breathing, swelling, and a feeling of fullness in the abdomen, and a general feeling of tiredness. These symptoms usually develop very quickly
These may be symptoms of a condition called “capillary leak syndrome” and can cause blood to leak from small blood vessels into other parts of your body and may require urgent medical attention.
- if you have any of the following side effects or a combination of them:
- fever, or chills, or feeling very cold, rapid heartbeat, confusion, or disorientation, difficulty breathing, severe pain or discomfort, and sweaty skin.
These may be symptoms of a condition called “sepsis” (also called “blood poisoning”), a severe infection with an inflammatory reaction throughout the body, which can be life-threatening and requires urgent medical attention.
- if you have pain in the upper left side of your abdomen (stomach), pain in the lower left side of your ribcage, or pain in the left shoulder tip, as these may be symptoms of an enlarged spleen (splenomegaly) or a possible rupture of the spleen.
- if you are being treated for severe chronic neutropenia and have blood in the urine (haematuria). Your doctor may perform regular urine tests if you have this side effect or if you have protein in the urine (proteinuria).
A common side effect of Neupogen is pain in the muscles or bones (musculoskeletal pain), which can be relieved by taking ordinary painkillers (analgesics). Patients undergoing stem cell or bone marrow transplantation may experience graft-versus-host disease (GVHD) - this is a reaction of the donor cells against the patient receiving the transplant, whose signs and symptoms include rash on the palms of the hands or soles of the feet, and ulcers and sores in the mouth, gut, liver, skin, or eyes, lungs, vagina, and joints.
In healthy stem cell donors, an increase in white blood cells in the blood (leucocytosis) and a decrease in platelets may be considered to reduce the ability of the blood to clot (thrombocytopenia), both will be monitored by your doctor.
Very common side effects(may affect more than 1 in 10 people):
- decrease in platelets, reducing the ability of the blood to clot (thrombocytopenia)
- low red blood cell count (anaemia)
- headache
- diarrhoea
- vomiting
- nausea
- unusual hair loss (alopecia)
- fatigue
- inflammation and swelling of the mucous membranes of the digestive tract (mucositis)
- fever (pyrexia)
Common side effects(may affect up to 1 in 10 people):
- lung inflammation (bronchitis)
- upper respiratory tract infection
- urinary tract infection
- decreased appetite
- difficulty sleeping (insomnia)
- dizziness
- loss of sensation, especially in the skin (hypoesthesia)
- tingling or numbness of the hands or feet (paraesthesia)
- low blood pressure (hypotension)
- high blood pressure (hypertension)
- cough
- coughing up blood (haemoptysis)
- pain in the mouth and throat (oropharyngeal pain)
- nosebleeds (epistaxis)
- constipation
- mouth ulcers
- enlargement of the liver (hepatomegaly)
- rash
- redness of the skin (erythema)
- muscle spasms
- pain when urinating (dysuria)
- chest pain
- pain
- general weakness (asthenia)
- feeling unwell (malaise)
- swelling of the hands and feet (peripheral oedema)
- increase in certain enzymes in the blood
- changes in blood biochemical parameters
- transfusion reactions
Uncommon side effects(may affect up to 1 in 100 people):
- increase in white blood cells in the blood (leucocytosis)
- allergic reactions (hypersensitivity)
- graft-versus-host disease (GVHD)
- high levels of uric acid in the blood, which can cause gout (hyperuricaemia)
- damage to the liver caused by a blockage in the small veins within the liver (veno-occlusive disease)
- abnormal functioning of the lungs, causing difficulty breathing (respiratory failure)
- swelling and/or fluid in the lungs (pulmonary oedema)
- inflammation of the lungs (interstitial lung disease)
- abnormal chest X-rays (pulmonary infiltrates)
- bleeding in the lungs (pulmonary haemorrhage)
- lack of oxygen in the lungs (hypoxia)
- rough skin rash (maculopapular rash)
- a disease that causes a decrease in bone density, making them weaker, more fragile, and more prone to breaking (osteoporosis)
- reaction at the injection site
Rare side effects(may affect up to 1 in 1,000 people):
- severe pain in the bones, chest, gut, or joints (sickle cell crisis)
- sudden life-threatening allergic reaction (anaphylactic reaction)
- pain and swelling of the joints, similar to gout (pseudogout)
- a change in the way the body regulates body fluids that can cause swelling (fluid volume alteration)
- inflammation of the blood vessels in the skin (cutaneous vasculitis)
- painful dark red ulcers on the limbs and sometimes on the face and neck, which are accompanied by fever (Sweet's syndrome)
- worsening of rheumatoid arthritis
- unusual change in urine
- decreased bone density
- inflammation of the aorta (the blood vessel that carries blood from the heart to the rest of the body), see section 2.
- formation of blood cells outside the bone marrow (extramedullary haematopoiesis)
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Neupogen
Keep this medicine out of the sight and reach of children.
Store in a refrigerator (2°C – 8°C).
Keep the container in the outer packaging to protect it from light.
Accidental freezing of Neupogen does not affect it.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled syringe or on the carton after EXP. The expiry date is the last day of the month shown.
Do not use this medicine if you notice discolouration, turbidity, or particles; it should be a clear and colourless liquid.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Neupogen
- The active ingredient is filgrastim 30 million units (0.6 mg/ml).
- The other components are sodium acetate, sorbitol (E420), polysorbate 80, and water for injectable preparations.
Appearance of the Product and Container Contents
Neupogen is a clear and colorless injectable solution (injectable) / concentrate for solution for infusion (sterile concentrate) in a pre-filled syringe.
Neupogen is available in packs of one or five pre-filled syringes. Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Marketing Authorization Holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer:
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer:
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
Local Representative of the Marketing Authorization Holder
Spain
Amgen, S.A.
Plaça del Gas, 1
Torre Marenostrum
Torre A, planta 20
08003 Barcelona
Tel: 93 600 18 60
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the name Neupogen, except in Cyprus, Greece, and Italy where it is called Granulokine.
Date of Last Revision of this Leaflet: March 2024
Other Sources of Information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Instructions for Injecting Neupogen
This section contains information on how to administer a Neupogen injection.
Important: do not attempt to inject yourself unless your doctor or nurse has taught you how to do it.
Neupogen is injected into the tissue just under the skin. This is called a subcutaneous injection.
Necessary Equipment
To administer a subcutaneous injection, you will need:
- a new pre-filled syringe of Neupogen; and
- cotton wool with alcohol or similar
What Should I Do Before Injecting Neupogen Subcutaneously?
- Remove the tray containing the syringe from the refrigerator and let it stand at room temperature for about 30 minutes, or gently hold it in your hands for a few minutes. This will make the injection less painful. Do notheat Neupogen in any other way (e.g., do not heat it in the microwave or in hot water).
- Do not shake the pre-filled syringe.
- Place the tray in your hand and remove the cover from the tray.
- Turn the tray over to place the pre-filled syringe in the palm of your hand.
- Do notremove the needle cover until you are ready for the injection.
- Check the expiration date on the label of the pre-filled syringe (EXP). Do not use it if it has passed the last day of the indicated month.
- Check the appearance of Neupogen. It should be a clear and colorless liquid. Do not use it if you notice discoloration, turbidity, or particles in it.
- Wash your hands carefully.
- Find a clean, comfortable, and well-lit surface and place all the necessary equipment within your reach.
How Do I Prepare the Neupogen Injection?
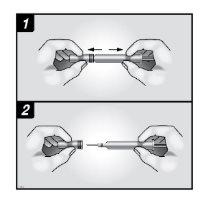
Before injecting Neupogen, you must do the following:
- To avoid bending the needle, firmly grasp the body of the pre-filled syringe. Gently pull the needle cover straight off without twisting, as shown in figures 1 and 2.
- Do not touch the needle or push the plunger.
- You may notice a small air bubble in the pre-filled syringe. Do not remove the air bubble before the injection. Injecting the solution with an air bubble is not harmful.
- The pre-filled syringe is now ready to use.
Where Should I Inject Neupogen?
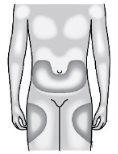
The most suitable places for injection are the top of the thighs and the abdomen. If someone else is injecting you, they can also inject you in the back of your arms.
You can change the injection site if you notice redness or swelling at the site.
How Do I Inject Neupogen?
- Disinfect the skin using cotton wool with alcohol and pinch the skin (without squeezing) between your thumb and index finger.
- Insert the needle fully into the skin as your nurse or doctor has taught you.
- Push the plunger with slow and constant pressure, keeping the skin pinched at all times until the syringe is empty.
- Remove the needle and release the skin.
- If you notice any blood, you can gently remove it with a little cotton wool or gauze. Do not rub the injection site. If necessary, you can cover the injection site with a bandage.
- Use each syringe for a single injection. Do not use any remaining Neupogen that may have been left in the syringe.
Remember:if you have any problems, do not hesitate to ask your doctor or nurse for help and advice.
Disposing of Used Syringes
- Do not put the protective cover back on used needles, as you could accidentally prick yourself.
- Keep used syringes out of the reach and sight of children.
- Syringes should not be thrown away in the trash. Your pharmacist will know how to dispose of used syringes or those that are no longer needed.
This information is intended only for healthcare professionals:
Neupogen should be diluted in 20 ml of a 5% glucose solution when used as a concentrate for solution for infusion. Please consult the Product Characteristics for additional information.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEUPOGEN 48 MU (0.96 mg/ml) PRE-FILLED SYRINGE SOLUTIONDosage form: INJECTABLE, 12 IU/0.2 mlActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.3 mg / prefilled syringeActive substance: filgrastimManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for NEUPOGEN 48 MU (0.96 mg/ml) PRE-FILLED SYRINGE SOLUTION
Discuss questions about NEUPOGEN 48 MU (0.96 mg/ml) PRE-FILLED SYRINGE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






