
AVANCORT 27.5 micrograms/spray NASAL SUSPENSION

How to use AVANCORT 27.5 micrograms/spray NASAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Avancort 27.5 micrograms/spray, nasal spray suspension
fluticasone furoate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Avancort and what is it used for
- What you need to know before you use Avancort
- How to use Avancort
- Possible side effects
- Storage of Avancort
- Contents of the pack and other information
1. What is Avancort and what is it used for
Avancort belongs to a group of medicines called glucocorticoids. Fluticasone furoate works by reducing the inflammation caused by allergies (rhinitis), and thus reduces the symptoms of the allergy.
Avancort nasal spray suspension is used to treat the symptoms of allergic rhinitis including nasal congestion, increased nasal secretion or nasal itching, sneezing, and runny or itchy eyes in adults and children 6 years and older.
Allergic symptoms may appear at certain times of the year due to allergy to plant or tree pollen (hay fever), or they may occur throughout the year due to allergy to animals, dust mites, or molds, among others.
2. What you need to know before you use Avancort
Do not use Avancort
- if you are allergic to fluticasone furoate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Avancort
Children and adolescents
Do not use in children under 6 years of age.
The use of Avancort:
- may cause a delay in the growth of children when used for long periods. The doctor will regularly check the child's height and ensure that the child receives the minimum effective dose.
- may cause eye disorders such as glaucoma (increased eye pressure) or cataracts (loss of lens transparency). Inform your doctor if you have had any of these disorders in the past or if you experience blurred vision or other visual disturbances while using fluticasone furoate.
Other medicines and Avancort
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription.
It is especially important that you inform your doctor if you are taking or have recently taken any of the following medicines:
- steroids in tablets or injected steroids
- steroids in creams
- medicines for asthma
- ritonavir or cobicistat, used in the treatment of AIDS
- ketoconazole, used in the treatment of fungal infections
Your doctor will assess whether you should take fluticasone furoate with these medicines. It is possible that your doctor may want to monitor you closely if you are taking any of these medicines, as they may increase the effects of Avancort.
Fluticasone furoate should not be used at the same time as other nasal sprays that contain steroids.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Do not use Avancort if you are pregnant, or are planning to become pregnant, unless your doctor or pharmacist advises you to do so.
Do not use Avancort if you are breastfeedingunless your doctor or pharmacist advises you to do so.
Driving and using machines
Avancort is unlikely to affect your ability to drive or use machines.
Avancort contains benzalkonium chloride
This medicine contains 8.25 micrograms of benzalkonium chloride per spray and 13.75 micrograms of polysorbate in each spray.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long-term treatment.
Polysorbates may cause allergic reactions.
Tell your doctor or pharmacist if you experience discomfort when using the spray.
3. How to use Avancort
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
When to use Avancort
- Use once a day.
- Use at the same time each day.
This medicine will treat your symptoms throughout the day and night.
How long it takes Avancort to work
Some people may not feel the full effects until several days after starting to use Avancort.
However, it is usually effective between 8 and 24 hours after use.
How much to use
Adults and children from 12 years
- The usual starting doseis 2 sprays in each nostril once a day.
- Once symptoms are under control, it may be possible to reduce the dose to 1 spray in each nostril, once a day.
Children from 6 to 11 years
- The usual starting doseis 1 spray in each nostril once a day.
- If symptoms are very severe, your doctor may increase the dose to 2 sprays in each nostril once a day, until symptoms are under control. After that, it may be possible to reduce the dose to 1 spray in each nostril once a day.
How to use the nasal spray
Avancort has almost no taste or smell. It is sprayed into the nose as a fine mist. Be careful not to spray the medicine into your eyes. If this happens, rinse your eyes with water.
There is a step-by-step guide on using the nasal spray after section 6 of this leaflet. Follow the guide carefully to get the full benefit of using Avancort.
See the step-by-step guide for using the nasal sprayafter section 6.
If you use more Avancort than you should
Consult your doctor or pharmacist.
If you forget to use Avancort
If you forget to take a dose, take it when you remember.
If it is almost time for your next dose, wait until then. Do not take a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, or if you experience discomfort when using the nasal spray, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions: seek medical help immediately
Allergic reactions to Avancort are rare and affect less than 1 in 1,000 people. In a small number of people, allergic reactions can become severe, even life-threatening if not treated. The symptoms include:
- wheezing, coughing, or difficulty breathing
- a sudden feeling of weakness or dizziness (leading to collapse or loss of consciousness)
- swelling of the face
- skin rash or redness.
In many cases, these symptoms will be signs of less serious side effects. But you must be aware that they can be potentially serious- so if you notice any of these symptoms:
Contact your doctor as soon as possible.
Very common side effects(may affect more than 1 in 10 people)
- Nosebleeds (usually minor), especially if you use Avancort for more than 6 weeks continuously.
Common side effects(may affect up to 1 in 10 people)
- Nasal ulcers - which can cause irritation or discomfort in your nose. You may notice blood when you blow your nose.
- Headache.
- Shortness of breath.
Uncommon side effects(may affect up to 1 in 100 people)
- Pain, burning, irritation, painful sensation, or dryness inside the nose.
Rare side effects(may affect up to 1 in 10,000 people)
- Small holes (perforations) in the nasal septum that separates the nostrils.
Frequency not known(frequency cannot be estimated from the available data)
- Slowing of growth in children.
- Blurred vision or temporary changes in vision with prolonged use.
- Chest tightness causing difficulty breathing.
- Voice disorder, loss of voice.
- Taste disorder, loss of taste, loss of smell.
The use of nasal corticosteroids may affect the normal production of hormones in your body, especially if high doses are used for long periods. In children, these side effects may cause slower growth.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Avancort
Keep this medicine out of the sight and reach of children.
It is preferable to store Avancort 27.5 micrograms/spray, nasal spray suspension in an upright position.
Always keep the cap on.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP:”. The expiry date is the last day of the month shown. Avancort should be used within 2 months after first opening.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to a pharmacy. Ask your pharmacist how to dispose of containers and any unused medicines. This will help protect the environment.
6. Contents of the pack and other information
Composition of Avancort
- The active substance is fluticasone furoate. Each spray delivers 27.5 micrograms of fluticasone furoate.
- The other ingredients are: glucose, microcrystalline cellulose (E460) and sodium carmellose (E466), polysorbate 80 (E433), benzalkonium chloride, disodium edetate, and purified water (see section 2).
Appearance of the product and pack contents
The medicine is a white nasal spray suspension contained in a 15 ml amber glass bottle with a metered-dose pump, a nasal applicator (spray nozzle), and a cap.
Avancort is available in one pack size: 1 bottle of 120 sprays.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) – Spain
Manufacturer
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) – Spain
Curida AS
Solbærvegen 5,
2409 Elverum,
Norway
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria: Fluticasonfuroat Goibela 27,5 Mikrogramm/Sprühstoß Nasenspray, Suspension
Spain: Avancort 27,5 micrograms/spray, suspensión para pulverización nasal
Date of last revision of this leaflet:March 2025
Other sources of information
Step-by-step guide for using the nasal spray.
STEP-BY-STEP GUIDE FOR USING THE NASAL SPRAY
Appearance of the nasal spray
The nasal spray is contained in an amber glass bottle. It will contain 120 sprays.
Five important things you need to know about using the nasal spray
- Avancort is contained in an amber glass bottle. If you need to check how much is left, hold the nasal spray upright to the light.This way you will be able to see the level through the window.
- When you use the nasal spray for the first time, you will need to shake it vigorouslywith the cap on for 10 seconds. This is important because Avancort is a dense suspension that becomes liquid when shaken well. It will only spray when it becomes liquid.
- You must press the dosing button firmlyand completely to release the mist through the nasal applicator - see drawing a.
- Always keep the cap onthe nasal spray when not in use. The cap prevents dust from entering, seals tightly under pressure, and prevents the nasal applicator from becoming blocked.
- Never use a pinor any other sharp object to unblock the nasal applicator. This would damage the nasal spray.
Preparing the nasal spray for use
You must prepare the nasal spray:
- before using it for the first time
- if you have left it uncapped for 5 days or have not used the nasal device for 30 days or more.
Preparing the nasal spray helps ensure that you always receive the full dose of medicine. Follow these steps:
- Shake the nasal spray vigorouslywith the cap on for 10 seconds.
- Remove the cap.
- Hold the nasal spray uprightand point the nasal applicator away from you.
- Press the dosing button firmlyand completely - see drawing a.Do this at least 6 timesuntil a fine spray mist is released into the air.
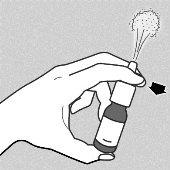 a
a
The nasal spray is now ready for use.
Using the nasal spray
- Shake the nasal sprayvigorously.
- Remove the cap.
- Blow your noseto clear your nostrils, then tilt your head slightly forward.
- Place the nasal applicator in one of your nostrils. Point the tip of the applicator slightly outward, away from the nasal septum. This helps the medicine reach the right part of the nose - see drawing b.
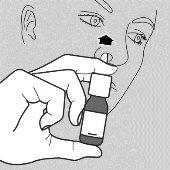 b
b
- Press the dosing button firmlyand completely, while breathing in through your nose.
- Remove the applicator and exhale through your mouth.
- If your dose is 2 sprays in each nostril, repeat steps 4 to 6.
- Repeat steps 4 to 7 in the other nostril.
- Replace the capon the nasal spray.
Cleaning the nasal spray
After each use:
- Wipe the nasal applicator with a clean, dry tissue.
- Do not use water to clean it.
- Never use a pin or any other sharp object in the nasal applicator.
- Always replace the caponce you have finished.
If the nasal spray does not seem to be working:
- Check that there is still medicine left. Look at the level through the window. If the level is very low, it may not be enough for the nasal spray to work.
- Check if the nasal spray has been damaged.
- If you think the nasal applicator may be blocked, do not use a pinor any other sharp object to unblock it.
- Try to restart it by following the instructions mentioned in “Preparing the nasal spray for use”.
- If it still does not work, or if a jet of liquid is produced, take the nasal spray to the pharmacy and consult your pharmacist.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
You can access detailed and up-to-date information about this medicine by scanning the QR code included in the leaflet and packaging with your smartphone. You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/89779/P_89779.html
QR code to: https://cima.aemps.es/cima/dochtml/p/89779/P_89779.html
- Country of registration
- Average pharmacy price6.99 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AVANCORT 27.5 micrograms/spray NASAL SUSPENSIONDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: NASAL PRODUCT, 27.5 micrograms/sprayActive substance: fluticasone furoateManufacturer: Teva B.V.Prescription requiredDosage form: NASAL PRODUCT, 27.5 micrograms/sprayActive substance: fluticasone furoateManufacturer: Cipla EuropePrescription required
Online doctors for AVANCORT 27.5 micrograms/spray NASAL SUSPENSION
Discuss questions about AVANCORT 27.5 micrograms/spray NASAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











