
METOJECT 10 mg/ 0.20 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use METOJECT 10 mg/ 0.20 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Metoject 7.5mg/0.15ml solution for injection in pre-filled syringe
Metoject 10mg/0.20ml solution for injection in pre-filled syringe
Metoject 12.5mg/0.25ml solution for injection in pre-filled syringe
Metoject 15mg/0.30ml solution for injection in pre-filled syringe
Metoject 17.5mg/0.35ml solution for injection in pre-filled syringe
Metoject 20mg/0.40ml solution for injection in pre-filled syringe
Metoject 22.5mg/0.45ml solution for injection in pre-filled syringe
Metoject 25mg/0.50ml solution for injection in pre-filled syringe
Metoject 27.5mg/0.55ml solution for injection in pre-filled syringe
Metoject 30mg/0.60ml solution for injection in pre-filled syringe
methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Metoject and what is it used for
- What you need to know before you use Metoject
- How to use Metoject
- Possible side effects
- Storage of Metoject
- Contents of the pack and other information
1. What is Metoject and what is it used for
Metoject contains methotrexate as the active substance.
Methotrexate is a substance with the following properties:
- it interferes with the growth of certain cells in the body that reproduce quickly,
- it reduces the activity of the immune system (the body's defense mechanism),
- it has anti-inflammatory effects.
Metoject is indicated for the treatment of:
- active rheumatoid arthritis in adult patients,
- severe active polyarticular forms of juvenile idiopathic arthritis, when the response to non-steroidal anti-inflammatory drugs (NSAIDs) has not been adequate,
- severe recalcitrant and disabling psoriasis that does not respond adequately to other treatments such as phototherapy, PUVA, and retinoids, and severe psoriatic arthritis in adult patients,
- mild to moderate Crohn's disease in adult patients when adequate treatment with other medications is not possible.
Rheumatoid arthritis (RA) is a chronic disease of the connective tissue, characterized by inflammation of the synovial membranes (joint membranes). These membranes produce a fluid that acts as a lubricant in many joints. Inflammation causes the membrane to thicken and the joint to swell.
Juvenile arthritis affects children and adolescents under 16 years of age. Polyarticular forms are indicated if there is involvement of 5 or more joints in the first 6 months of the disease.
Psoriatic arthritis is a type of arthritis with psoriatic lesions on the skin and nails, especially in the joints of the fingers and toes.
Psoriasis is a chronic and frequent skin disease, characterized by red patches covered with thick, dry, silvery, and adherent scales.
Metoject modifies and slows down the progression of the disease.
Crohn's disease is a type of inflammatory bowel disease that can affect any part of the gastrointestinal tract, causing symptoms such as abdominal pain, diarrhea, vomiting, or weight loss.
2. What you need to know before you use Metoject
Do not use Metoject:
- if you are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6),
- if you have severe liver or kidney disease or blood disorders,
- if you regularly drink large amounts of alcohol,
- if you have a severe infection, such as tuberculosis, HIV, or other immunodeficiency syndromes,
- if you have ulcers in the mouth, stomach ulcers, or intestinal ulcers,
- if you are pregnant or breastfeeding (see section "Pregnancy, breastfeeding, and fertility"),
- if you are receiving live vaccines at the same time.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Metoject if:
- you are elderly or generally feel unwell and weak,
- you have altered liver function,
- you have dehydration problems (fluid loss).
- you have diabetes mellitus and are being treated with insulin.
Special precautions for treatment with Metoject
Methotrexate temporarily affects the production of sperm and eggs, which is reversible in most cases. Methotrexate can cause abortions and severe birth defects. If you are a woman, you must avoid becoming pregnant while using methotrexate and for at least 6 months after finishing treatment. If you are a man, you must avoid fathering a child if you are receiving methotrexate and for at least 3 months after the end of treatment. See also section "Pregnancy, breastfeeding, and fertility".
Recommended follow-up tests and precautions
Even if methotrexate is used in low doses, serious side effects can occur. To detect them in time, your doctor will need to perform tests and analytical controls.
Before starting treatment
Before starting treatment, you will have blood tests to check that you have enough blood cells. You will also have blood tests to check liver function and to find out if you have hepatitis. In addition, serum albumin (a blood protein), hepatitis status, and kidney function will be checked. Your doctor may also decide to perform other liver tests; some of these may involve imaging of the liver, and others may require taking a small tissue sample from the liver to examine it in more detail. Your doctor may also check if you have tuberculosis, and you may have a chest X-ray or a lung function test.
During treatment
Your doctor may perform the following tests:
- Examination of the oral cavity and pharynx to identify changes in the mucous membrane, such as inflammation or ulceration.
- Blood tests/blood cell counts with cell count and measurement of serum methotrexate levels.
- Blood tests to monitor liver function.
- Imaging tests to monitor liver function.
- Removal of a small tissue sample from the liver to examine it in more detail.
- Blood tests to monitor kidney function.
- Monitoring of the respiratory tract and, if necessary, a lung function test.
It is very important that you attend these scheduled tests.
If the results of any of these tests are abnormal, your doctor will adjust your treatment accordingly.
Elderly patients
Elderly patients treated with methotrexate should be closely monitored by a doctor to detect possible side effects as soon as possible.
The deterioration of liver and kidney function related to age and low body reserves of folic acid in old age require a relatively low dose of methotrexate.
Other precautions
Acute pulmonary hemorrhage has been reported with methotrexate in patients with underlying rheumatological disease. If you notice blood when coughing or spitting, you should contact your doctor immediately.
Methotrexate can affect the immune system and the results of vaccination. It can also affect the results of immunological tests. It can exacerbate chronic inactive infections (e.g., herpes zoster [shingles], tuberculosis, hepatitis B or C). During treatment with Metoject, you should not receive live vaccines.
Methotrexate can make the skin more sensitive to sunlight. Avoid intense sunlight and do not use sunbeds or UV lamps without medical advice. To protect your skin from intense sunlight, wear suitable clothing or use a sunscreen with a high protection factor.
During treatment with methotrexate, radiation-induced dermatitis and sunburn (memory reactions) may recur. Psoriatic lesions may worsen during UV radiation and concurrent administration of methotrexate.
An increase in the size of lymph nodes (lymphoma) may occur, and in such cases, treatment should be discontinued.
Diarrhea can be a toxic effect of Metoject that requires discontinuation of treatment. If you have diarrhea, talk to your doctor.
Certain brain disorders (encephalopathy/leukoencephalopathy) have been reported in cancer patients treated with methotrexate. It cannot be ruled out that these side effects may occur when methotrexate is used to treat other diseases.
If you, your partner, or your caregiver notice the appearance or worsening of neurological symptoms, such as general muscle weakness, vision changes, changes in thinking, memory, and orientation that cause confusion, and changes in personality, contact your doctor immediately because these may be symptoms of a rare and serious brain infection called progressive multifocal leukoencephalopathy (PML).
Other medicines and Metoject
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. Also, keep this in mind for medicines that you may take in the future.
The effect of treatment may be affected if Metoject is administered at the same time as certain medicines:
- Antibiotics such as tetracyclines, chloramphenicol, non-absorbable broad-spectrum antibiotics, penicillins, glycopeptides, sulfonamides, ciprofloxacin, and cefalotin (medicines to prevent or treat certain infections).
- Non-steroidal anti-inflammatory drugs (NSAIDs) or salicylates (medicines for pain or inflammation, such as acetylsalicylic acid, diclofenac, or ibuprofen, or pyrazolones).
- Metamizole (synonyms novaminsulfon and dipyrone) (medicine for severe pain and/or fever).
- Probenecid (medicine for gout).
- Weak organic acids such as loop diuretics.
- Medicines that can cause adverse effects on the bone marrow, such as trimethoprim-sulfamethoxazole (an antibiotic) and pyrimethamine.
- Other medicines used to treat rheumatoid arthritis, such as leflunomide, sulfasalazine, and azathioprine.
- Cyclosporin (to suppress the immune system).
- Mercaptopurine (a cytostatic).
- Retinoids (medicines for psoriasis and other skin diseases).
- Theophylline (medicine for bronchial asthma and other lung diseases).
- Certain medicines for stomach problems, such as omeprazole and pantoprazole.
- Hypoglycemic agents (medicines used to lower blood sugar levels).
Vitamins containing folic acidmay alter the effect of your treatment and should only be taken when advised by your doctor.
Vaccination with live vaccines should be avoided.
Using Metoject with food, drinks, and alcohol
During treatment with Metoject, you should avoid consuming alcohol and large amounts of coffee, caffeinated soft drinks, and black tea.
Pregnancy, breastfeeding, and fertility
Pregnancy
Do not use Metoject during pregnancy or if you are trying to become pregnant. Methotrexate can cause birth defects, harm the fetus, or cause abortions. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that methotrexate is not administered to pregnant patients or those planning to become pregnant.
In women of childbearing age, any possibility of pregnancy should be excluded with appropriate measures, such as a pregnancy test before starting treatment.
You should avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment, using reliable contraceptive methods during this time (see also section "Warnings and precautions").
If you become pregnant during treatment or suspect that you may be pregnant, consult your doctor as soon as possible. You should be offered information about the risk of harmful effects on the child during treatment.
If you wish to become pregnant, consult your doctor, who may refer you to a specialist for information before the planned start of treatment.
Breastfeeding
Stop breastfeeding before and during treatment with Metoject.
Male fertility
Available data do not indicate an increased risk of malformations or abortions if the father takes a dose of methotrexate less than 30 mg/week. However, this risk cannot be completely ruled out. Methotrexate can be genotoxic, which means it can cause genetic mutations. Methotrexate can affect sperm production and cause birth defects. Therefore, you should avoid fathering a child or donating sperm while taking methotrexate and for at least 3 months after stopping treatment.
Driving and using machines
Treatment with Metoject can cause side effects that affect the central nervous system, such as fatigue and dizziness. Therefore, the ability to drive or use machines may be affected in certain cases. If you feel tired or drowsy, do not drive or use machines.
Metoject contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use Metoject
Important dosage warning for Metoject (methotrexate):
Use Metoject only once a weekfor the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, and Crohn's disease. Excessive use of Metoject (methotrexate) can be fatal. Read section 3 of this leaflet carefully. If you have any doubts, consult your doctor or pharmacist before using this medicine.
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
Your doctor will determine the dose, which will be adjusted individually. The treatment usually takes between 4 and 8 weeks to take effect.
Metoject will be administered by or under the supervision of your doctor or healthcare professional in the form of an injection under the skin (subcutaneous injection) only once a week. Together with your doctor, you will choose a day of the week that is suitable for you to receive the injection.
Use in children and adolescents
The doctor decides what dose is suitable for children and adolescents with polyarticular forms of juvenile idiopathic arthritis.
Metoject is not recommended for use in children under 3 years of age due to limited experience in this age group.
Duration and method of administration
Metoject is injected subcutaneously once a week.
The treating doctor will decide the duration of treatment. Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris, psoriatic arthritis, and Crohn's disease with Metoject is long-term treatment.
At the start of treatment, Metoject may be injected by medical personnel. However, your doctor may decide that you can learn to inject Metoject yourself under the skin. You will receive the necessary training for this.
Under no circumstances should you attempt to inject yourself unless you have been taught to do so.
See the instructions for use at the end of the leaflet.
The handling and disposal of the product will be done according to the guidelines for other cytotoxic preparations, in accordance with local regulations. Pregnant healthcare personnel should not handle or administer Metoject.
Methotrexate should not come into contact with the skin or mucous membranes. If it does, the affected area should be rinsed immediately with plenty of water.
If you use more Metoject than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use Metoject
Do not take a double dose to make up for forgotten doses.
If you stop treatment with Metoject
If you stop treatment with Metoject, consult your doctor immediately.
If you think the effect of Metoject is too strong or too weak, consult your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The frequency and severity of adverse effects will depend on the dose and frequency of administration. It is essential that your doctor performs periodic checks, as severe adverse effects can occur even with the lowest doses. Your doctor will perform tests to control abnormalitiesthat occur in the blood (such as low levels of white blood cells, low platelets, and lymphoma) and changes in the kidneys and liver.
Inform your doctor immediatelyif you experience any of the following symptoms, as they may indicate a severe or life-threatening adverse effect that may require urgent specific treatment:
- Dry cough, without expectoration, difficulty breathing, and fever; may be signs of lung inflammation [frequent]
- Blood when coughing or spitting; may be signs of pulmonary hemorrhage [frequency not known]
- Symptoms of liver damage, such as yellowing of the skin or eyes; methotrexate can cause chronic liver damage (cirrhosis), scar tissue formation in the liver (hepatic fibrosis), fatty liver degeneration [all infrequent], acute liver inflammation (hepatitis) [rare], and liver failure [very rare]
- Allergic reaction symptoms, such as skin rash including itching and redness of the skin, swelling of the hands, feet, ankles, face, lips, mouth, and throat (which can cause difficulty swallowing or breathing) and feeling of fainting; these may be signs of severe allergic reactions or anaphylactic shock [rare]
- Symptoms of kidney damage, such as inflammation of the hands, ankles, or feet, or changes in the frequency of urination or decreased (oliguria) or absent (anuria) urine output; these may be signs of renal failure [rare]
- Infection symptoms, such as fever, chills, muscle pain, sore throat; methotrexate can make you more susceptible to infections. Severe infections, such as a type of pneumonia (Pneumocystis jirovecii pneumonia) or blood infection (sepsis), can occur [rare]
- Symptoms such as weakness on one side of the body (stroke) or pain, swelling, redness, and unusual heat in one leg (deep vein thrombosis); this can happen when a blood clot breaks loose and blocks a blood vessel (thromboembolic event) [rare]
- Fever and severe deterioration of your general condition, or sudden fever accompanied by sore throat or mouth problems, or urinary problems; methotrexate can cause a sudden drop in the number of certain white blood cells (agranulocytosis) and severe bone marrow suppression [very rare]
- Unexpected bleeding, e.g., bleeding gums, blood in the urine, vomiting blood, or bruising; these may be signs of a severe decrease in the number of platelets caused by severe bone marrow depression episodes [very rare]
- Symptoms such as severe headache, often in combination with fever, stiff neck, nausea, vomiting, disorientation, and sensitivity to light, may indicate inflammation of the brain membranes (aseptic meningitis) [very rare]
- Certain brain disorders (encephalopathy/leukoencephalopathy) have been reported in cancer patients treated with methotrexate; these adverse effects cannot be ruled out when methotrexate treatment is used to treat other diseases; signs of this type of brain disorder may be altered mental state, movement disorders (ataxia), visual disorders, or memory disorders [frequency not known]
- Severe skin rash or blistering of the skin (this can also affect the mouth, eyes, and genitals); these may be signs of a condition called Stevens-Johnson syndrome or toxic epidermal necrolysis/Lyell syndrome [very rare]
The following are other adverse effects that may occur:
Very common:may affect more than 1 in 10 people
- Inflammation of the mouth lining, indigestion, nausea, loss of appetite, abdominal pain.
- Abnormal liver function test results (ASAT, ALAT, bilirubin, alkaline phosphatase).
Common:may affect up to 1 in 10 people
- Mouth ulcers, diarrhea.
- Skin rash, redness of the skin, itching.
- Headache, fatigue, drowsiness.
- Decreased blood cell production with a decrease in the number of white blood cells, red blood cells, or platelets.
Uncommon:may affect up to 1 in 100 people
- Inflammation of the throat.
- Inflammation of the intestine, vomiting, pancreatitis, black or tarry stools, gastrointestinal ulcers, and bleeding.
- Reactions similar to sunburn due to increased skin sensitivity to sunlight, hair loss, increased number of rheumatoid nodules, skin ulcers, shingles, blood vessel inflammation, herpes-like rash, hives.
- Onset of diabetes mellitus.
- Dizziness, confusion, depression.
- Decreased serum albumin.
- Decreased number of all blood cells and platelets.
- Inflammation and ulceration of the urinary bladder or vagina, decreased renal function, urinary disorders.
- Joint pain, muscle pain, reduced bone mass.
Rare:may affect up to 1 in 1,000 people
- Inflammation of the gum tissue.
- Increased skin pigmentation, acne, bruising of the skin due to bleeding from the blood vessels (ecchymosis, petechiae), allergic inflammation of the blood vessels.
- Decreased number of antibodies in the blood.
- Infection (including reactivation of inactive chronic infections), red eyes (conjunctivitis).
- Mood changes (mood alterations).
- Visual disturbances.
- Inflammation of the sac around the heart, fluid accumulation in the sac around the heart, obstruction of cardiac filling due to fluid in the sac surrounding the heart.
- Low blood pressure.
- Scarring of lung tissue (pulmonary fibrosis), difficulty breathing, and bronchial asthma, fluid accumulation in the sac surrounding the lung.
- Stress fracture.
- Electrolyte disturbances.
- Fever, altered wound healing.
Very rare:may affect up to 1 in 10,000 people
- Toxic and acute dilation of the intestine (toxic megacolon).
- Increased nail pigmentation, inflammation of the cuticles (acute paronychia), deep infection of the hair follicles (furunculosis), visible enlargement of small blood vessels.
- Local injury at the injection site (formation of sterile abscesses, changes in fatty tissue).
- Pain, loss of strength, or sensation of numbness or tingling/sensitivity to stimuli less than normal, altered taste (metallic taste), convulsions, paralysis, meningism.
- Visual disturbance, non-inflammatory eye disorder (retinopathy).
- Loss of sexual appetite, impotence, enlarged male breasts, altered sperm formation (oligospermia), menstrual disorders, vaginal discharge.
- Increased size of lymph nodes (lymphoma).
- Lymphoproliferative disorders (excessive increase in white blood cells).
Frequency not known:cannot be estimated from the available data
- Increased number of certain white blood cells.
- Nasal bleeding.
- Protein in the urine.
- Feeling of weakness.
- Jawbone injury (secondary to excessive increase in white blood cells).
- Tissue destruction at the injection site.
- Redness and peeling of the skin.
- Swelling.
Subcutaneous administration of methotrexate is well-tolerated locally. Only mild local skin reactions were observed, which decreased during treatment.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Metoject
Keep this medicine out of the sight and reach of children.
Store below 25°C.
Store the pre-filled syringes in the outer packaging to protect them from light.
Do not use this medicine after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and unused medicines in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition ofMetoject
- The active ingredient is methotrexate. 1 ml of solution contains methotrexate disodium corresponding to 50 mg of methotrexate.
- The other components are sodium chloride, sodium hydroxide, and water for injectable preparations.
Appearance of the product and container contents
The Metoject pre-filled syringes contain a clear yellow-brown solution.
For containers containing syringes with a safety system
The syringe is equipped with a safety system to prevent needlestick injuries and reuse of the needle.
The following package sizes are marketed:
Pre-filled graduated syringes with subcutaneous injection needles attached, packaged in blisters, containing 0.15 ml, 0.20 ml, 0.25 ml, 0.30 ml, 0.35 ml, 0.40 ml, 0.45 ml, 0.50 ml, 0.55 ml, and 0.60 ml of injectable solution available in packages of 1, 4, 5, 6, 10, 11, 12, and 24 pre-filled syringes with a safety system.
Pre-filled graduated syringes with subcutaneous injection needles attached, packaged in blisters, containing 0.15 ml, 0.20 ml, 0.25 ml, 0.30 ml, 0.35 ml, 0.40 ml, 0.45 ml, 0.50 ml, 0.55 ml, and 0.60 ml of injectable solution available in calendar packages of 6 and 12 pre-filled syringes with a safety system.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
medac Gesellschaft für klinische Spezialpräparate mbH
Theaterstr. 6
22880 Wedel
Germany
Tel.: +49 4103 8006-0
Fax: +49 4103 8006-100
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Laboratorios Gebro Pharma, S.A.
Avenida Tibidabo n° 29
08022 Barcelona
Spain
Tel. +34 93 205 86 86
This medication is authorized in the member states of the European Economic Area under the following names:
Austria, Belgium, Czech Republic, Finland, Greece, Hungary, Iceland, Netherlands, Slovakia, Slovenia, Spain, Sweden: Metoject
Germany, Denmark, Estonia, Latvia, Lithuania, Norway, Poland, and Portugal: Metex
Italy: Reumaflex
Date of the last revision of this leaflet: August 2024
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
You can access detailed and updated information on how to administer this medication by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet addresses:
Metoject 7.5 mg/0.15 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/71107
Metoject 10 mg/0.20 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/71108
Metoject 12.5 mg/0.25 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/73716
Metoject 15 mg/0.30 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/71109
Metoject 17.5 mg/0.35 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/73717
Metoject 20 mg/0.40 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/71110
Metoject 22.5 mg/0.45 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/73718
Metoject 25 mg/0.50 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/71111
Metoject 27.5 mg/0.55 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/73719
Metoject 30 mg/0.60 ml solution for injection in pre-filled syringe:
https://cima.aemps.es/info/72384
Instructions for use for subcutaneous administration
Metoject is administered as an injection under the skin once a week exclusively. Read the instructions carefully before starting to administer the injection, and always use the application technique advised by your doctor, nurse, or pharmacist.
If you have any problems or questions, contact your doctor, nurse, or pharmacist.
Preparation
Select a clean, flat, and well-lit work surface.
Wash your hands carefully.
Open the box containing the pre-filled syringe of methotrexate and read the leaflet carefully. Remove the pre-filled syringe from the package at room temperature.
Before using it, check the Metoject syringe for visible defects (or cracks). If there is a small air bubble visible in the solution, this will not affect your dose or cause you any harm.
Injection site

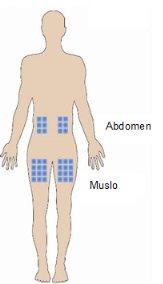
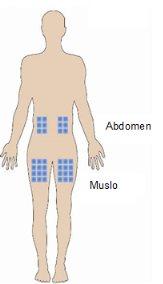
The best places for injection are:
- the upper part of the thigh,
- the abdomen, except for the area around the navel.
- If someone is helping you with the injection, you can also apply the injection to the back of the arm, just below the shoulder.
- Change the injection site each time. This can reduce the risk of developing irritations at the injection site.
- Never apply the injection to painful, bruised, reddened, hardened, or scarred skin. If you have psoriasis, do not attempt to inject directly into lesions or patches of elevated, thickened, reddened, or scaly skin.
Injection of the solution
- Select an injection site and clean the selected site and surrounding area with water and soap or disinfectant.
- Remove the protective plastic cap
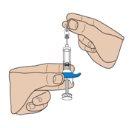
Remove the gray plastic cap from the needle by pulling it to separate it from the syringe. If you have difficulty removing the cap, gently twist it with an outward movement.
Important: Do nottouch the needle of the pre-filled syringe.
Note: Once the cap is removed, administer the injection without delay.
- Insertion of the needle
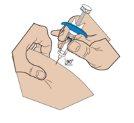
With two fingers, form a fold in the skin and quickly insert the needle at a 90-degree angle.
- Injection
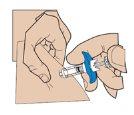
Insert the needle fully into the skin fold. Slowly push the plunger and inject the liquid under the skin. Hold the skin firmly until the injection is complete.
Remove the needle carefully in a straight line.
- Dispose of the used syringe, including the needle, in a sharps container. Do not throw them in household waste.
Methotrexate should not come into contact with the skin surface or mucous membranes. If this happens, rinse immediately with plenty of water.
If you or someone in your environment is injured with the needle, consult your doctor immediately and do not use this pre-filled syringe.
Elimination and other handling
Handling and disposal of the medication and pre-filled syringe will be carried out in accordance with local regulations. Pregnant healthcare personnel should not handle or administer Metoject.
For containers containing syringes with a safety system
Instructions for use for subcutaneous administration
Metoject is administered as an injection under the skin once a week exclusively. Read the instructions carefully before starting to administer the injection, and always use the application technique advised by your doctor, nurse, or pharmacist.
If you have any problems or questions, contact your doctor, nurse, or pharmacist.
Preparation
Select a clean, flat, and well-lit work surface.
Wash your hands carefully.
Open the box containing the pre-filled syringe of methotrexate with a safety system and read the leaflet carefully. Remove the pre-filled syringe from the package at room temperature.
Before using it, check the Metoject syringe for visible defects (or cracks). If there is a small air bubble visible in the solution, this will not affect your dose or cause you any harm.
Injection site
The best places for injection are:
- the upper part of the thigh,
- the abdomen, except for the area around the navel.
- If someone is helping you with the injection, you can also apply the injection to the back of the arm, just below the shoulder.
- Change the injection site each time. This can reduce the risk of developing irritations at the injection site.
- Never apply the injection to painful, bruised, reddened, hardened, or scarred skin. If you have psoriasis, do not attempt to inject directly into lesions or patches of elevated, thickened, reddened, or scaly skin.
Injection of the solution
- Select an injection site and clean the selected site and surrounding area with water and soap or disinfectant.
- Remove the protective plastic cap
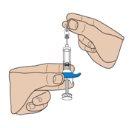
Remove the gray plastic cap from the needle by pulling it to separate it from the syringe. If you have difficulty removing the cap, gently twist it with an outward movement.
Important: Do not touch the needle of the pre-filled syringe.
Note: Once the cap is removed, administer the injection without delay.
- Insertion of the needle
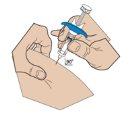
With two fingers, form a fold in the skin and quickly insert the needle at a 90-degree angle.
- Injection
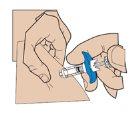
Insert the needle fully into the skin fold. Slowly push the plunger and inject the liquid under the skin.
- Removal of the needle
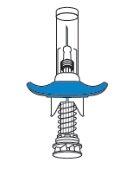
Hold the skin firmly until the injection is complete.
Remove the needle carefully in a straight line.
A protective cap will automatically enclose the needle.
Note: The protection system that is activated by releasing the protective cap can only be activated when the syringe is completely empty by pushing the plunger to the bottom.
- Dispose of the used syringe, including the needle, in a sharps container. Do not throw them in household waste.
Methotrexate should not come into contact with the skin surface or mucous membranes. If this happens, rinse immediately with plenty of water.
If you or someone in your environment is injured with the needle, consult your doctor immediately and do not use this pre-filled syringe.
Elimination and other handling
Handling and disposal of the medication and pre-filled syringe will be carried out in accordance with local regulations. Pregnant healthcare personnel should not handle or administer Metoject.
- Country of registration
- Average pharmacy price13.49 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METOJECT 10 mg/ 0.20 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for METOJECT 10 mg/ 0.20 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about METOJECT 10 mg/ 0.20 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






