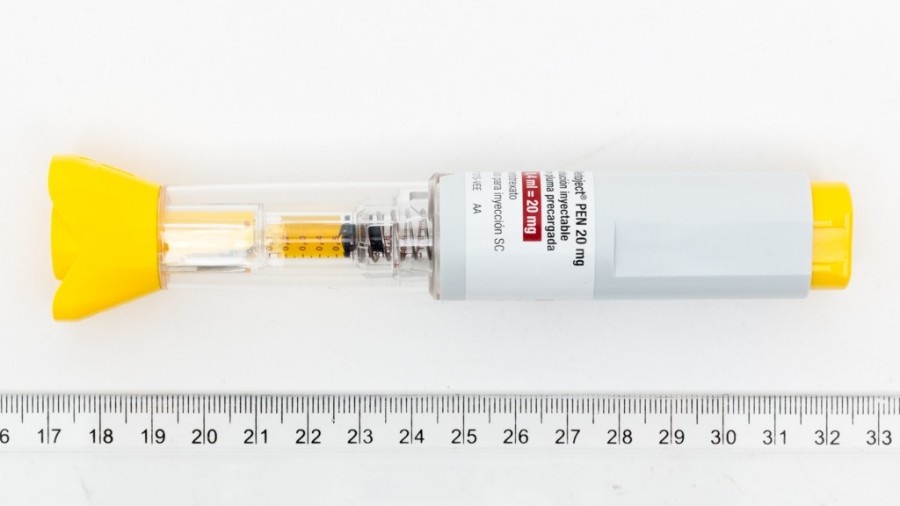
METHOFILL PEN 20 mg/0.40 ml INJECTABLE SOLUTION IN PRE-FILLED PEN EFG

How to use METHOFILL PEN 20 mg/0.40 ml INJECTABLE SOLUTION IN PRE-FILLED PEN EFG
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Methofill Pen 20 mg/0.40 ml Solution for Injection in Pre-filled Pen EFG
methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Methofill Pen is and what it is used for
- What you need to know before you use Methofill Pen
- How to use Methofill Pen
- Possible side effects
- Storage of Methofill Pen
- Contents of the pack and other information
1. What Methofill Pen is and what it is used for
This medicine is indicated for the treatment of:
- active rheumatoid arthritis in adult patients,
- severe active polyarticular juvenile idiopathic arthritis, when the response to non-steroidal anti-inflammatory drugs (NSAIDs) has been inadequate,
- moderate to severe psoriasis in adult patients, and severe psoriatic arthritis in adults,
- mild to moderate Crohn's disease in adult patients when adequate treatment with other medicines is not possible.
Rheumatoid arthritis (RA) is a chronic disease of the connective tissue, characterized by inflammation of the synovial membranes (joint membranes). These membranes produce a fluid that acts as a lubricant in many joints. Inflammation causes the membrane to thicken and the joint to swell.
Juvenile arthritis affects children and adolescents under 16 years of age. Polyarticular forms are indicated if there is involvement of 5 or more joints in the first 6 months of the disease.
Psoriasis is a chronic and frequent skin disease, characterized by red patches covered with thick, dry, silvery, and adherent scales.
Psoriatic arthritis is a type of arthritis with psoriatic lesions on the skin and nails, especially in the joints of the fingers and toes.
This medicine modifies and slows down the progression of the disease.
Crohn's disease is a type of inflammatory bowel disease that can affect any part of the gastrointestinal tract, causing symptoms such as abdominal pain, diarrhea, vomiting, or weight loss.
2. What you need to know before you use Methofill Pen
Do not use Methofill Pen
- if you are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6),
- if you have severe liver or kidney disease or blood disorders,
- if you regularly drink large amounts of alcohol,
- if you have a severe infection, such as tuberculosis, HIV, or other immunodeficiency syndromes,
- if you have oral ulcers, stomach ulcers, or intestinal ulcers,
- if you are pregnant or breastfeeding (see section "Pregnancy, breastfeeding, and fertility"),
- if you are receiving live vaccines at the same time.
Warnings and precautions
Methotrexate can make your skin more sensitive to sunlight. Avoid intense sunlight and do not use sunbeds or UV lamps without medical advice. To protect your skin from intense sunlight, wear suitable clothing or use a sunscreen with a high protection factor.
Consult your doctor or pharmacist before starting to use this medicine if:
- you are elderly or generally feel unwell and weak,
- you have liver problems,
- you have dehydration problems (loss of fluids).
Acute pulmonary hemorrhage has been reported with methotrexate in patients with underlying rheumatologic disease. If you cough up blood or spit up blood, you should contact your doctor immediately.
Special precautions for treatment with Methofill Pen
Methotrexate temporarily affects the production of sperm and eggs, which is reversible in most cases. Methotrexate can cause abortions and severe birth defects. If you are a woman, you should avoid becoming pregnant if you are receiving methotrexate and for at least 6 months after stopping treatment with methotrexate. If you are a man, you should avoid fathering a child if you are receiving methotrexate and for at least 3 months after stopping treatment. See also section "Pregnancy, breastfeeding, and fertility".
Recommended follow-up tests and safety measures:
Even when this medicine is administered at low doses, serious side effects can occur. To detect them in time, it is necessary for your doctor to perform blood tests and check-ups.
Before starting treatment with Methofill Pen:
Before starting treatment, you will have blood tests to check that you have enough blood cells, and tests to check liver function and to find out if you have hepatitis. Additionally, the concentration of serum albumin (a blood protein) will be checked, the state of hepatitis (liver infection) and kidney function. Your doctor may also decide to perform other liver tests, some of which may involve imaging of your liver and others may require taking a small tissue sample from your liver to examine it more closely. Your doctor will also check if you have tuberculosis (an infectious disease with small nodules in the affected tissue) and will perform a chest X-ray or a lung function test.
During treatment:
Your doctor will perform the following tests:
- examination of the oral cavity and pharynx to detect changes in the mucous membrane, such as inflammation or ulceration
- blood count with number of blood cells and measurement of serum methotrexate levels
- blood tests to monitor liver function
- imaging tests to monitor liver condition
- small tissue sample taken from the liver to examine it more closely
- blood tests to monitor kidney function
- review of the respiratory system and, if necessary, lung function test
It is very important that you attend these scheduled tests.
If the results of any of these tests are remarkable, your doctor will adjust your treatment accordingly.
Methotrexate can affect the immune system and the results of vaccination. It can also affect the results of immunological tests. It can reactivate chronic inactive infections (such as herpes zoster ["shingles"], tuberculosis, hepatitis B or C). During treatment with this medicine, you should not receive live vaccines.
During treatment with methotrexate, radiation-induced dermatitis and sunburn (memory reactions) may reappear. Psoriatic lesions may worsen during UV radiation and simultaneous administration of methotrexate.
An increase in the size of lymph nodes (lymphoma) may occur, and in such cases, treatment should be discontinued.
Diarrhea can be a side effect of this medicine that requires discontinuation of treatment. If you have diarrhea, talk to your doctor.
Certain brain disorders (encephalopathy/leukoencephalopathy) have been reported in cancer patients treated with methotrexate. It cannot be ruled out that these side effects may occur when methotrexate is used to treat other diseases.
If you, your partner, or your caregiver notice the onset or worsening of neurological symptoms, such as general muscle weakness, vision changes, changes in thinking, memory, and orientation that cause confusion and changes in personality, contact your doctor immediately because these may be symptoms of a rare and serious brain infection called progressive multifocal leukoencephalopathy (PML).
Elderly patients
Elderly patients treated with methotrexate should be closely monitored by a doctor to detect any possible side effects as soon as possible.
The deterioration of liver and kidney function related to age, as well as the low body reserves of folic acid in the elderly, require a relatively low dose of methotrexate.
Using Methofill Pen with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Keep this in mind also for medicines that you may take in the future.
The effect of treatment may be affected if this medicine is administered at the same time as certain medicines:
- Antibioticssuch as: tetracyclines, chloramphenicol, non-absorbable broad-spectrum antibiotics, penicillins, glycopeptides, sulfonamides, ciprofloxacin, and cefalotin (medicines to prevent or combat certain infections).
- Amoxicillin(penicillins may reduce the excretion of methotrexate, causing a potential increase in side effects).
- Non-steroidal anti-inflammatory drugsor salicylates(medicines for pain or inflammation such as acetylsalicylic acid, diclofenac, and ibuprofen or pyrazolones)
- Probenecid(medicine for gout).
- Weak organic acids such as loop diuretics
- Medicines that can cause side effects on the bone marrow, such as trimethoprim-sulfamethoxazole (an antibiotic) and pyrimethamine
- Other medicines used to treat rheumatoid arthritissuch as leflunomide, sulfasalazine, and azathioprine.
- Mercaptopurine (a cytostaticmedicine).
- Retinoids (medicines for psoriasisand other dermatological diseases)
- Theophylline (medicine for bronchial asthmaand other pulmonary diseases)
- Some medicines for stomach problemssuch as omeprazole and pantoprazole.
- Hypoglycemics (medicines used to lower blood sugar levels).
Metamizol (synonyms novaminsulfon and dipyrone)(medicine for severe pain and/or fever);
Vitamins containing folic acidmay alter the effect of your treatment and should only be taken when advised by your doctor.
Vaccination with live vaccines should be avoided.
Using Methofill Pen with food, drinks, and alcohol
During treatment with this medicine, you should avoid consuming alcohol and large amounts of coffee, soft drinks containing caffeine, and black tea.
Pregnancy, breastfeeding, and fertility
Pregnancy
Do not use this medicine during pregnancy or if you are trying to become pregnant. Methotrexate can cause birth defects, harm the fetus, or cause abortions. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that methotrexate is not administered to pregnant patients or those planning to become pregnant. In women of childbearing age, any possibility of pregnancy should be excluded with appropriate measures, for example, a pregnancy test before starting treatment. You should avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment, using reliable contraceptive methods during this time (see also section "Warnings and precautions").
If you become pregnant during treatment or suspect that you may be pregnant, consult your doctor as soon as possible. You should be offered information about the risk of harmful effects to the child during treatment.
If you wish to become pregnant, consult your doctor, who may refer you to a specialist for information before the planned start of treatment.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to become pregnant, consult your doctor or pharmacist before using this medicine.
Breastfeeding
Stop breastfeeding before and during treatment with this medicine.
Male fertility
Available data do not indicate a higher risk of malformations or abortions if the father takes a dose of methotrexate less than 30 mg/week. However, this risk cannot be completely ruled out. Methotrexate can be genotoxic, which means it can cause genetic mutations. Methotrexate can affect sperm production and cause birth defects. Therefore, you should avoid fathering a child or donating sperm while taking methotrexate and for at least 3 months after stopping treatment.
Driving and using machines
Treatment with this medicine can cause side effects that affect the central nervous system, such as fatigue and dizziness. Therefore, the ability to drive or use machines may be affected in certain cases. If you feel tired or drowsy, do not drive or use machines.
Methofill Pen contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per dose; this is essentially "sodium-free".
3. How to use Methofill Pen
Follow exactly the administration instructions of this medication indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Your doctor will determine the dose, which will be adjusted individually. Normally, the treatment takes between 4 and 8 weeks to take effect.
The injection of this medication is administered subcutaneously (under the skin) by or under the supervision of a doctor or healthcare professional onlyonce a week. Along with your doctor, you will choose a day of the week that is suitable for you to receive the injection.
At the beginning of the treatment, this medication may be injected by medical personnel. However, it is possible that your doctor decides that you can learn to inject Methofill Pen yourself. You will receive the necessary training for this. Under no circumstances should you attempt to inject yourself unless you have been taught to do so.
Important warning about the dose of Methofill Pen (methotrexate): Use Methofill Pen only once a weekfor the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, and psoriatic arthritis, and Crohn's disease. Excessive use of Methofill Pen (methotrexate) can be fatal. Read section 3 of this prospectus carefully. If you have any doubts, consult your doctor or pharmacist before using this medication. |
Use in children and adolescents
The doctor decides what the appropriate dose is for children and adolescents with polyarticular forms of juvenile idiopathic arthritis.
This medication is not recommended for use in children under 3 years of age because experience is limited in this age group.
Duration and administration
This medication is injected once a week.
The attending physician will decide the duration of the treatment. The treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris, psoriatic arthritis, and Crohn's disease with this medication is long-term treatment.
At the beginning of the treatment, this medication may be injected by medical personnel. However, it is possible that your doctor decides that you can learn to inject Methofill Pen yourself. You will receive the necessary training for this.
Under no circumstances should you attempt to inject yourself unless you have been taught to do so.
You can also find guidance on how to use this medication in the "Instructions for use" section.
Note that the entire contents must be used.
The handling and disposal of the medication and the pre-filled pen will be carried out in accordance with local regulations. Pregnant healthcare personnel should not handle or administer this medication.
Methotrexate should not come into contact with the skin surface or mucous membranes. If it comes into contact, the affected area should be rinsed immediately with plenty of water.
Instructions for use
Recommendations
- Read the instructions carefully before starting to administer the injection.
- Always use the application technique advised by your doctor, nurse, or pharmacist.
Additional information
The handling and disposal of the medication and the pre-filled pen will be carried out in accordance with local regulations. Pregnant healthcare personnel should not handle or administer this medication.
Methotrexate should not come into contact with the skin surface or mucous membranes. If it comes into contact, the affected area should be rinsed immediately with plenty of water.
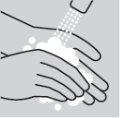
What to do before administering your injection
- Select a clean, flat, and well-lit work surface.
- Check the expiration date. Do not use if the expiration date has passed.
- Take an alcohol swab and a container for disposing of sharp objects.
- Open the box containing the pre-filled pen of methotrexate and place the pre-filled pen on a flat and clean surface (such as a table). Read the prospectus carefully.
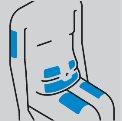
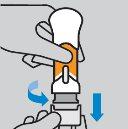
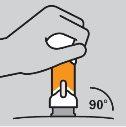
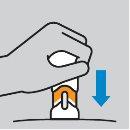
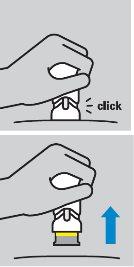
How to prepare the injection
|
|
|
|
|
|
|
|
|
|
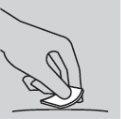
How to inject
|
|
|
|
|
|
|
|
Methotrexate should not come into contact with the skin surface or mucous membranes. In case of contamination, the affected area should be rinsed immediately with plenty of water.
Who to contact in case of need
If you have any doubts or problems, contact your doctor, pharmacist, or nurse.
If you or someone around you is injured with the needle, consult your doctor immediately and do not use this pre-filled syringe.
Disposal and other handling
The handling and disposal of the medication and the pre-filled pen will be carried out in accordance with local regulations. Pregnant healthcare personnel should not handle or administer methotrexate.
If you use more Methofill Pen than you should
If you use more medication than you should, consult your doctor immediately.
If you forget to use Methofill Pen
Do not use a double dose to make up for forgotten doses.
If you interrupt treatment with Methofill Pen
If you interrupt treatment with this medication, consult your doctor immediately.
If you feel that the effect of this medication is too strong or too weak, consult your doctor or pharmacist.
If you suspect that you (or someone else) have administered too much Methofill Pen, contact your doctor or go to the nearest hospital immediately, or consult the Toxicology Information Service, phone 91 562 04 20. They will decide what measures to take based on the severity of the poisoning. Bring the medication with you if you go to the doctor or hospital.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The frequency and severity of adverse effects will depend on the dose and frequency of administration. It is essential that your doctor performs periodic checks, as severe adverse effects can occur even with the lowest doses. Your doctor will perform tests to detect anomaliesthat occur in the blood (such as low white blood cell count, low platelet count, or lymphoma) and alterations in the kidneys and liver.
If you experience any of the following symptoms, contact your doctor immediately, as they may indicate a severe, potentially life-threatening adverse effect that requires urgent specific treatment:
- Dry persistent cough, without expectoration, difficulty breathing, and fever;may be signs of lung inflammation [frequent]
- Blood when coughing or spitting;may be signs of pulmonary hemorrhage [unknown frequency]
- Symptoms of liver damage, such as yellowing of the skin or whites of the eyes;methotrexate can cause chronic liver injury (liver cirrhosis), scarring of liver tissue (liver fibrosis), fatty degeneration of the liver [all infrequent], acute liver inflammation (hepatitis) [rare], and liver failure [very rare]
- Allergic symptoms, such as skin rash including itching, swelling of hands, feet, ankles, face, lips, mouth, or throat (which can cause difficulty swallowing or breathing), and feeling of fainting;these may be signs of severe allergic reactions or anaphylactic shock [rare]
- Symptoms of kidney damage, such as swelling of hands, ankles, or feet, or changes in urination frequency or decreased (oliguria) or absent (anuria) urine;these may be signs of kidney failure [rare]
- Symptoms of infections, e.g., fever, chills, pain, sore throat;methotrexate can make you more prone to infections. Severe infections such as a certain type of pneumonia (Pneumocystis jirovecii pneumonia) or septicemia (sepsis) [rare] can occur
- Symptoms such as weakness on one side of the body (stroke) or pain, swelling, redness, and unusual heat in one leg (deep vein thrombosis);this can happen when a blood clot breaks loose and causes a blockage of a blood vessel (thromboembolic event) [rare]
- Fever and severe deterioration of your general condition, or sudden fever accompanied by sore throat or mouth pain, or urinary problems;methotrexate can cause a sharp drop in the number of certain white blood cells (agranulocytosis) and severe bone marrow suppression [very rare]
- Unexpected bleeding, e.g., bleeding gums, blood in urine, vomiting blood, or bruising;these may be signs of a severe decrease in platelet count caused by severe episodes of bone marrow depression [very rare]
- Symptoms such as severe headache, often in combination with fever, stiff neck, nausea, vomiting, disorientation, and sensitivity to light;may indicate inflammation of the brain membranes (aseptic meningitis) [very rare]
- Certain brain disorders (encephalopathy/leukoencephalopathy) have been reported in cancer patients treated with methotrexate; these adverse effects cannot be ruled out when methotrexate treatment is used to treat other diseases; signs of this type of brain disorder may be altered mental state, movement disorders (ataxia), visual disorders, or memory disorders[unknown frequency]
- Severe skin rash or blistering of the skin (this can also affect the mouth, eyes, and genitals);these may be signs of a condition called Stevens-Johnson syndrome or toxic epidermal necrolysis [very rare]
The following are other adverse effects that may occur:
Very common:may affect more than 1 in 10 people
- Inflammation of the mouth lining, indigestion, nausea, loss of appetite, abdominal pain.
- Abnormal results in liver function tests (ASAT, ALAT, bilirubin, alkaline phosphatase).
Common:may affect up to 1 in 10 people
- Mouth ulcers, diarrhea.
- Rash, skin redness, itching.
- Headache, fatigue, drowsiness.
- Decreased blood cell production with decreased white blood cell, red blood cell, or platelet count
Uncommon:may affect up to 1 in 100 people
- Inflammation of the throat.
- Inflammation of the intestine, vomiting, pancreas inflammation, black or tarry stools, gastrointestinal ulcers, and bleeding.
- Reactions similar to sunburn due to increased skin sensitivity to sunlight, hair loss, increased number of rheumatoid nodules, skin ulcers, shingles, blood vessel inflammation, herpes-like rash, hives.
- Onset of diabetes mellitus.
- Dizziness, confusion, depression.
- Decreased serum albumin.
- Decreased number of all blood cells and platelets.
- Inflammation and ulcers of the urinary bladder or vagina, decreased kidney function, urinary disorders.
- Pain in the joints, muscle pain, reduced bone mass.
Rare:may affect up to 1 in 1,000 people
- Inflammation of gum tissue.
- Increased skin pigmentation, acne, bruising on the skin due to bleeding from blood vessels (ecchymosis, petechiae), allergic inflammation of blood vessels.
- Decreased number of antibodies in the blood.
- Infection (including reactivation of inactive chronic infections), red eyes (conjunctivitis).
- Mood changes (mood alterations).
- Visual disturbances.
- Inflammation of the sac around the heart, fluid accumulation in the sac around the heart, obstruction of heart filling due to fluid in the sac surrounding the heart.
- Low blood pressure.
- Scarring of lung tissue (pulmonary fibrosis), breathing difficulties, and bronchial asthma, fluid accumulation in the sac surrounding the lung.
- Stress fracture.
- Electrolyte disturbances.
- Fever, altered wound healing.
Very rare:may affect up to 1 in 10,000 people
- Toxic and acute dilation of the intestine (toxic megacolon).
- Increased nail pigmentation, inflammation of the cuticles (acute paronychia), deep infection of hair follicles (furunculosis), visible enlargement of small blood vessels.
- Pain, loss of strength, or numbness/tingling sensation, altered taste (metallic taste), seizures, paralysis, meningism.
- Visual disturbances, non-inflammatory eye disorder (retinopathy).
- Loss of sexual appetite, impotence, breast enlargement in males, altered sperm formation (oligospermia), menstrual disorders, vaginal discharge.
- Increased size of lymph nodes (lymphoma).
- Lymphoproliferative disorders (excessive increase in white blood cells).
Unknown frequency:cannot be estimated from available data
- Increased number of certain white blood cells.
- Nosebleeds.
- Protein in urine.
- Feeling of weakness.
- Damage to the jawbone (secondary to excessive increase in white blood cells).
- Tissue destruction at the injection site.
- Redness and peeling of the skin.
- Swelling.
Subcutaneous administration of methotrexate is well-tolerated locally. Only mild local skin reactions (such as burning sensations, erythema, swelling, color change, severe itching, pain) were observed, which decreased during treatment.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Methofill Pen
Keep this medicine out of the sight and reach of children.
Store below 30 °C.
Keep the pre-filled pens in the outer packaging to protect them from light.
Do not use this medicine after the expiration date stated on the carton and on the pre-filled pen after CAD. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package contents and additional information
Composition of Methofill Pen
- The active ingredient is methotrexate. 1 ml of solution contains methotrexate disodium equivalent to 50 mg of methotrexate.
1 pre-filled pen with 0.40 ml of solution contains 20 mg of methotrexate
- The other ingredients are sodium chloride, sodium hydroxide for pH adjustment, and water for injectable preparations.
Appearance and packaging of the product
Methofill Pen pre-filled pens contain a clear yellow-brown solution. The following pack sizes are available:
Pre-filled syringes containing 0.15 ml, 0.20 ml, 0.25 ml, 0.30 ml, 0.35 ml, 0.40 ml, 0.45 ml, 0.50 ml, 0.55 ml, and 0.60 ml of injectable solution, available in packs of 1 or multipacks of 4 (4 packs of 1) pre-filled pens.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Accord Healthcare S.L.U.
World Trade Center
Moll de Barcelona, s/n
Edifici Est 6ª planta,
08039 Barcelona,
Spain
Manufacturer:
Accord Healthcare Polska Sp.z.o.o.
ul.Lutomierska 50
pabianice, 95-200
Poland
or
Laboratori Fundació DAU
C/ C, 12-14 Pol. Ind. Zona Franca, Barcelona
Spain
Local representative:
Laboratorios Rubió, S.A.
Industria 29
Polígono Industria Comte de Sert
08755 Castellbisbal
(Barcelona)
Spain
Date of last revision of this leaflet: August 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
- Country of registration
- Average pharmacy price106.47 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METHOFILL PEN 20 mg/0.40 ml INJECTABLE SOLUTION IN PRE-FILLED PEN EFGDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for METHOFILL PEN 20 mg/0.40 ml INJECTABLE SOLUTION IN PRE-FILLED PEN EFG
Discuss questions about METHOFILL PEN 20 mg/0.40 ml INJECTABLE SOLUTION IN PRE-FILLED PEN EFG, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions