MEPACT 4 mg POWDER FOR PERFUSION SUSPENSION


How to use MEPACT 4 mg POWDER FOR PERFUSION SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
MEPACT 4 mg powder for concentrate for dispersion for infusion.
mifamurtide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you experience side effects, consult your doctor, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is MEPACT and what is it used for
- What you need to know before you start using MEPACT
- How to use MEPACT
- Possible side effects
- Storage of MEPACT
- Contents of the pack and further information
1. What is MEPACT and what is it used for
MEPACT contains the active substance mifamurtide, similar to a component of the cell wall of some bacteria. It stimulates the immune system to help your body destroy cancer cells.
MEPACT is used for the treatment of osteosarcoma (bone cancer) in children, adolescents, and young adults (between 2 and 30 years). It is used after surgery to remove the tumor and chemotherapy to destroy any remaining cancer cells and reduce the risk of cancer recurrence.
2. What you need to know before you start using MEPACT
Do not use MEPACT:
- if you are allergic (hypersensitive) to mifamurtide or any of the other ingredients of this medicine (listed in section 6).
- if you are using medicines that contain cyclosporin or other calcineurin inhibitors or high doses of non-steroidal anti-inflammatory drugs (NSAIDs) (see also "Use of other medicines").
Warnings and precautions
Consult your doctor before starting MEPACT:
- if you have or have had heart or blood vessel problems, such as blood clots (thrombosis), bleeding (hemorrhages), or inflammation of the veins (vasculitis). During MEPACT treatment, you will be closely monitored. If your symptoms do not disappear over time or worsen, you should contact your doctor, as it may be necessary to delay or stop MEPACT treatment.
- if you have a history of asthma or other respiratory disorders. Before using MEPACT, consult your doctor about whether you should continue using asthma medications while using MEPACT.
MEPACT.
- if you have a history of inflammatory or autoimmune disease or have been treated with corticosteroids or other medications that may affect your immune system.
- If you have any allergic reaction to medications, including rash, shortness of breath, and high blood pressure. If you experience worsening symptoms, you should contact your doctor, as these may have been caused by MEPACT.
- If you have stomach problems, such as nausea, vomiting, and loss of appetite. If your problems increase, you should contact your doctor, as these may have been caused by MEPACT when used with chemotherapy.
- If you develop chills or shaking, or feel hot. You should take your temperature, as you may have a fever. The presence of fever with a low white blood cell count (neutropenia) can be a sign of serious infection.
In section 4, detailed information is included on warnings and precautions related to side effects that may occur while taking this medicine.
Children
Do not give this medicine to children under 2 years of age because there is no information on its safety and efficacy in this age group.
Other medicines and MEPACT
Tell your doctor if you are using, have recently used, or may need to use any other medicine, including those obtained without a prescription. It is very important that you inform your doctor if you are using medicines that contain any of the following substances:
- cyclosporin, tacrolimus, used after a transplant to prevent organ rejection, and other immunosuppressants used, for example, to treat psoriasis (a skin disease).
- non-steroidal anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid, ibuprofen, or diclofenac, used to treat headaches, fever, or pain. Do not use MEPACT if you are using NSAIDs in high doses.
- corticosteroids, for the treatment of inflammation, allergies, or asthma. Regular use of corticosteroids should be avoided when using MEPACT, as it may affect how the medications work.
It is recommended to separate the administration times of MEPACT and doxorubicin or other medications if they are combined in the same chemotherapy regimen.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
MEPACT has not been tested in pregnant women. Therefore, MEPACT should not be used during pregnancy or in women of childbearing age who do not use an effective contraceptive method. Use an effective contraceptive method if you receive MEPACT treatment.
It is not known whether MEPACT passes into breast milk. If you are breastfeeding, consult your doctor.
Driving and using machines
Some very common or common side effects of MEPACT treatment (such as dizziness, vertigo, fatigue, and blurred vision) may affect your ability to drive and use machines.
MEPACT contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per dose unit; it is essentially "sodium-free".
3. How to use MEPACT
Dose and duration of treatment
MEPACT will be administered only under the supervision of a specialist doctor. Follow exactly the administration instructions of this medicine indicated by your doctor. If in doubt, consult your doctor again.
The recommended dose of MEPACT is 2 mg/m2 of body surface area. It will be administered twice a week (with at least three days apart) for the first 12 weeks and then once a week for another 24 weeks.
The treatment program may be adjusted to fit your chemotherapy schedule. You do not need to interrupt the MEPACT program if your chemotherapy is delayed; you will need to complete 36 weeks (9 months) of MEPACT treatment without interruption.
How MEPACT is administered
The lyophilized powder must be reconstituted in a liquid suspension, filtered using the provided filter, and then diluted before use. The MEPACT infusion is administered directly into a vein (intravenously) over approximately 1 hour. This will be carried out by your doctor or a nurse, who will also monitor you during this time. You do not need to be hospitalized to receive MEPACT. It can also be administered to outpatients.
If you use more MEPACT than you should
You may experience more serious side effects, such as fever, chills, fatigue, nausea, vomiting, headache, high blood pressure, or low blood pressure. In case of overdose, contact your doctor or go to the nearest hospital.
If you stop MEPACT treatment
Do not stop MEPACT treatment before completing the treatment program without consulting your doctor first. If you have any further questions on the use of this product, ask your doctor.
4. Possible side effects
Like all medicines, MEPACT can cause side effects, although not everybody gets them.
Most patients experienced chills, fever, and fatigue, especially during the first administration of MEPACT. These effects are usually mild to moderate and transient and can be treated by your doctor; for example, with paracetamol for fever. When used with chemotherapy, MEPACT treatment can often cause stomach problems, such as nausea, vomiting, and loss of appetite.
Consult your doctor immediately:
- if fever or chills persist for more than 8 hours after receiving the MEPACT dose, as it may indicate an infection, or
- if you experience a rash or have other respiratory problems (wheezing) or
- if you experience any stomach problems.
Very common side effects(may affect more than 1 in 10 people):
- fever, chills/shivering, weakness, fatigue, or general malaise
- nausea and/or vomiting, diarrhea, or constipation
- headache or dizziness
- rapid heart rate (tachycardia)
- high or low blood pressure
- loss of appetite
- sweating
- pain, which can be general pain, muscle and/or joint pain, and back, chest, abdominal, arm, or leg pain
- cough, breathing difficulties, or shortness of breath
- low body temperature
- low red blood cell count
Common side effects(may affect up to 1 in 10 people):
- bluish discoloration of tissues, such as skin or gums, due to lack of oxygen
- noticeable increase in heart rate or force of heartbeats
- swelling in arms or legs, or other sites
- chest discomfort
- stomach upset, loss of appetite, or weight loss
- injection site reactions, such as redness, swelling, infection, or other local reactions at the injection site or catheter insertion site.
- erythema or redness, skin inflammation, itching, dryness, paleness, or transient redness
- inflammation of skin, tendons, muscles, or similar tissues that support body structure
- vein inflammation
- abdominal pain in the upper or thoracic wall; abdominal distension or pain; indigestion or liver pain
- other types of pain, also in the neck, shoulder, groin, bones, or throat; postoperative pain
- muscle spasms or stiffness
- feeling of cold
- fatigue, dizziness, or somnolence
- burning sensation, itching, tingling, reduced sensitivity, or sensation without stimuli
- involuntary muscle jerks
- dehydration
- low potassium levels in the blood
- mucosal inflammation
- nasal, throat, or sinus congestion or inflammation
- upper respiratory tract infections (such as a cold) or urinary tract infections (such as a bladder infection)
- generalized infection
- Herpes simplex virus infection
- productive cough, wheezing, or breathing difficulties on exertion or worsening
- runny nose or nasal bleeding
- fluid in the lung cavity
- blood in the urine, difficulty or pain when urinating, or frequent urination
- difficulty sleeping, depression, anxiety, or confusion
- dizziness
- ringing in the ears
- blurred vision
- hair loss
- difficult or painful menstruation
- hearing loss
- low white blood cell count with or without fever, low platelet count
Frequency not known(cannot be estimated from available data):
- excessive fluid accumulation around the heart (pericardial effusion)
Reporting of side effects
If you experience any side effects, consult your doctor, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of MEPACT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the pack after "EXP".
The expiry date is the last day of the month stated.
Unopened vial:
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the vial in the outer packaging to protect it from light.
Reconstituted suspension:
Once reconstituted with a 9 mg/ml (0.9%) sodium chloride solution, store at room temperature (approximately 20°C - 25°C) and use within 6 hours. Do not use this medicine if you notice visible signs of deterioration.
Medicines should not be disposed of via wastewater or household waste. This will help protect the environment.
6. Container contents and additional information
What MEPACT contains
- The active substance is mifamurtide. Each vial contains 4 mg of mifamurtide. After reconstitution, each ml contains 0.08 mg of mifamurtide.
- The other components are palmitoyl-2-oleyl-sn-glycero-3-phosphocholine (POPC) and
1,2-dioleoyl-sn-glycero-3-phospho-L-serine monosodium salt (MOPS). See section 2 "MEPACT contains sodium".
Appearance of MEPACT and container contents
MEPACT is a white to off-white powder or mass for concentrate for dispersion for infusion.
MEPACT is presented in a cardboard box containing:
- A 50 ml vial with a grey butyl rubber stopper, aluminium seal and plastic flip-off cap.
- A sterile filter supplied in a blister.
Marketing authorisation holder:
Takeda France SAS
112 avenue Kléber
75116 Paris
France
Manufacturer:
Takeda Austria GmbH
St. Peter-Straße 25
A-4020 Linz
Austria
Delpharm Novara S.r.l.
Via Crosa, 86
28065 Cerano (NO)
Italy
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
-------------------------------------------------------------------------------------------------------------------------
---
The following information is intended exclusively for healthcare professionals:
Instructions for preparation of MEPACT for intravenous infusion
Materials supplied in each box -
- 1 vial of MEPACT (mifamurtide)
- 1 filter for MEPACT
Materials required but not supplied -
- Sodium chloride 9 mg/ml (0.9%) injection solution, 100 ml bag
- Sterile 60 or 100 ml syringe for single use with luer lock
- 2 sterile medium-calibre (18) injection needles
It is recommended that the reconstitution of the liposomal suspension be performed in a laminar flow cabinet using sterile gloves and aseptic techniques.
The lyophilised powder should be allowed to reach a temperature of approximately 20°C - 25°C before reconstitution, filtration using the provided filter and dilution. This takes about 30 minutes.
- Remove the seal from the vial and clean the stopper using a cotton swab moistened with alcohol.
- Remove the filter from the blister and remove the needle cap from the filter.
Then insert the needle into the vial, firmly piercing the stopper until it is securely in place. At this time, do not remove the luer connector cap from the filter.
- Unpack the bag with 100 ml of sodium chloride 9 mg/ml (0.9%) injection solution, the needle and the syringe (not supplied in the box).
- Clean the injection site of the sodium chloride 9 mg/ml (0.9%) injection solution bag with a cotton swab moistened with alcohol.
- Using the needle and syringe, withdraw 50 ml of sodium chloride 9 mg/ml (0.9%) injection solution from the bag.
- After removing the needle from the syringe, it should be attached to the filter by opening the luer connector cap of the filter (Figure 1).
|
- Add the sodium chloride 9 mg/ml (0.9%) injection solution to the vial through slow but firm pressure on the syringe plunger. Do not remove the filter or syringe from the vial.
- Allow the vial to stand for 1 minute to ensure thorough hydration of the dry substance.
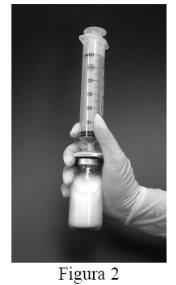
- Then, shake the vial vigorously for 1 minute while keeping the filter and syringe attached.During this time, the liposomes form spontaneously (Figure 2).
|
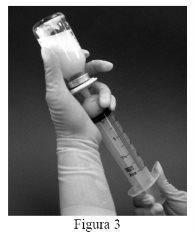
- The required dose can be withdrawn from the vial by inverting it and slowly withdrawing the syringe plunger (Figure 3). After reconstitution, each ml of suspension contains 0.08 mg of mifamurtide. The volume of suspension to be withdrawn is calculated as follows:
Volume to be withdrawn = [12.5 x calculated dose (mg)] ml
For ease of use, the following conversion table is provided:
Dose | Volume |
1.0 mg | 12.5 ml |
2.0 mg | 25 ml |
3.0 mg | 37.5 ml |
4.0 mg | 50 ml |
|
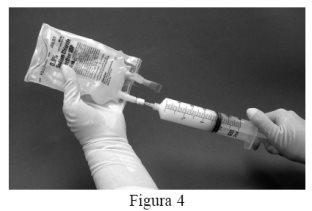
- Then remove the syringe from the filter and attach a new needle to the syringe containing the suspension. Clean the injection site of the sodium chloride 9 mg/ml (0.9%) injection solution bag with a cotton swab moistened with alcohol and inject the suspension from the syringe into the original bag containing the remaining 50 ml of sodium chloride 9 mg/ml (0.9%) injection solution (Figure 4).
|
- Gently turn the bag several times to mix the solution.
- Add the patient's identification, time and date to the label of the bag containing the reconstituted and diluted liposomal suspension.
- Chemical and physical stability has been demonstrated during use for 6 hours at room temperature (approximately between 20°C - 25°C).
- From a microbiological point of view, the product should be used immediately. If it is not to be used immediately, the storage times and conditions of the product once opened are the responsibility of the user and normally should not exceed 6 hours at room temperature.
No special requirements for disposal.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MEPACT 4 mg POWDER FOR PERFUSION SUSPENSIONDosage form: INJECTABLE, UnknownActive substance: tasonerminManufacturer: Belpharma S.A.Prescription requiredDosage form: INJECTABLE, 0.5 mg histamine dihydrochloride/0.5 mLActive substance: histamine dihydrochlorideManufacturer: Laboratoires DelbertPrescription requiredDosage form: INJECTABLE, 20 mg glatiramer acetate / mlActive substance: glatiramer acetateManufacturer: Teva GmbhPrescription required
Online doctors for MEPACT 4 mg POWDER FOR PERFUSION SUSPENSION
Discuss questions about MEPACT 4 mg POWDER FOR PERFUSION SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions