MENOPUR 600 IU POWDER AND SOLVENT FOR INJECTION

How to use MENOPUR 600 IU POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
MENOPUR 600 International Units powder and solvent for solution for injection
Highly purified menotropin
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack
- What Menopur is and what it is used for
- What you need to know before you use Menopur
- How to use Menopur
- Possible side effects
- Storage of Menopur
- Contents of the pack and other information
1. What Menopur is and what it is used for
Menopur contains menotropin (also called human menopausal gonadotropin or hMG-HP). It is a highly purified extract of the urine of postmenopausal women, and contains two hormones called follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH are present in both men and women, and help the reproductive organs to function normally.
Menopur is indicated for the treatment of infertility in the following situations:
- Women who cannot become pregnant because their ovaries do not produce eggs (including polycystic ovary syndrome). Menopur is used in women who have been treated with a medicine called clomiphene citrate to treat their infertility, but this medicine has not worked.
- Women in assisted reproduction programs (ART) (including in vitro fertilization/embryo transfer [IVF/ET], gamete intrafallopian transfer [GIFT], intracytoplasmic sperm injection [ICSI]). Menopur helps the ovaries to develop many egg sacs (follicles) where an egg can develop (multiple follicular development).
- Infertility in men with hypogonadotropic or normogonadotropic hypogonadism: in combination with human chorionic gonadotropin to stimulate spermatogenesis.
to stimulate sperm production.
2. What you need to know before you use Menopur
Before starting treatment with Menopur, you and your partner should be evaluated by a doctor to determine the causes of the infertility problem. In particular, you should be checked for the following diseases so that you can receive the most appropriate treatment:
- Malfunction of the thyroid gland and adrenal cortex
- High levels of a hormone called prolactin (hyperprolactinemia)
- Tumors in the pituitary gland (a gland located at the base of the brain)
- Tumors in the hypothalamus (an area located under the part of the brain called the thalamus).
If you know you have any of the diseases listed above, please inform your doctor before starting treatment with Menopur.
Do not use Menopur if:
- you are allergic (hypersensitive) to menotropin or any of the other components of Menopur listed in section 6.
In women:
In men:
- If you have prostate cancer
- If you have a tumor in the testicles.
Warnings and precautions
If you have:
- Abdominal pain
- Abdominal swelling
- Nausea
- Vomiting
- Diarrhea
- Weight gain
- Difficulty breathing
- Decreased urine output.
Consult your doctor directly, even if the symptoms develop a few days after the last injection was given. These can be signs of high levels of activity in the ovaries that can become serious.
If these symptoms become severe, the infertility treatment should be discontinued and you should receive treatment in a hospital.
By maintaining the recommended dose and careful monitoring of the treatment, the options for experiencing these symptoms will be reduced.
If you stop usingMenopur you may still experience these symptoms. Please contact your doctor immediately if you suffer from any of these symptoms.
While you are being treated with this medicine, your doctor will normally have you undergo ultrasound scansand sometimes blood teststo monitor your response to treatment.
When being treated with hormones like Menopur, the risk of:
- Ectopic pregnancy (pregnancy outside the uterus) if you have a history of fallopian tube disease
- Miscarriage
- Multiple pregnancy (twins, triplets, etc.)
- Congenital malformations (physical defects present in the baby at birth).
Some women who have been treated with several medicines for infertility have developed tumors in the ovaries and other reproductive organs. It is not yet known if treatment with hormones like this medicine causes these problems.
It is more likely that blood clots will form inside blood vessels (veins or arteries) in pregnant women. Infertility treatment may increase the likelihood of this happening, especially if you are overweight or have a known blood clotting disorder (thrombophilia) or if you or a relative have had blood clotting problems. Inform your doctor if you think this applies to you.
Use in children
Menopur is not indicated for use in children.
Use of Menopur with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No studies of interactions with other medicines have been performed with Menopur in humans.
When treating infertile men, menotropin and human chorionic gonadotropin can be administered at the same time.
Clomiphene citrate is another medicine used in the treatment of infertility. If Menopur is used at the same time as clomiphene citrate, the effect on the ovaries may be increased.
Menopur can be used at the same time as Bravelle (another medicine used to treat infertility). Please see section 3 "How to use Menopur".
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Menopur is not indicated in any case for the treatment of pregnant or breastfeeding women.
Driving and using machines
It is very rare that Menopur affects the ability to drive and use machines.
Important information about some of the components of Menopur:
Menopur contains less than 1 mmol of sodium chloride (23 mg) per dose, and is therefore essentially sodium-free.
3. How to use Menopur
Follow the instructions for administration of Menopur indicated by your doctor.
In case of doubt, consult your doctor or pharmacist again.
- Women who do not ovulate (do not produce eggs):
Treatment will start within the first 7 days of the menstrual cycle (day 1 is the first day of the period). Treatment should be administered every day for at least 7 days.
The initial dose is usually 75-150 IU per day. This dose may be increased according to your response to treatment up to a maximum of 225 IU per day. A single dose should be administered at least 7 days before the dose is adjusted by the doctor. The recommended dose increase is 37.5 IU per adjustment (and no more than 75 IU). The treatment cycle should be abandoned if there is no adequate response after 4 weeks.
When an optimal response is obtained, a single injection of another hormone called human chorionic gonadotropin (hCG) will be administered, at a dose of 5,000 to 10,000 IU, 1 day after the last dose of Menopur. It is recommended to have intercourse on the same day as the administration of hCG and the following day. Alternatively, intrauterine insemination (injection of sperm directly into the uterus) can be performed. Your doctor should follow your progress very closely for at least 2 weeks after the administration of hCG.
Your doctor will monitor the effect of Menopur treatment. Depending on the progress, your doctor will decide to discontinue treatment with Menopur and not administer the hCG injection. In this case, you should use a contraceptive method (condom) or not have sexual intercourse until the next period begins.
ii. Women in assisted reproduction programs:
If you are also receiving treatment with GnRH agonists (a medicine that helps the hormone called Gonadotropin-Releasing Hormone (GnRH) to work), treatment with Menopur should start approximately 2 weeks after starting GnRH agonist treatment.
If you are also receiving treatment with GnRH antagonists, treatment with Menopur should start on day 2 or 3 of the menstrual cycle (day 1 is the first day of the period).
Menopur will be administered daily for at least 5 days. The recommended initial dose of Menopur is 150-225 International Units. This dose may be increased according to your response to treatment up to a maximum of 450 International Units per day. The dose should not be increased by more than 150 IU per adjustment. Treatment is not usually recommended for more than 20 days.
If there are enough egg sacs, a single injection of a medicine called human chorionic gonadotropin (hCG) will be administered at a dose of up to 10,000 IU to induce ovulation (release of an egg).
Your doctor should follow your progress very closely for at least 2 weeks after the administration of hCG.
Your doctor will monitor the effect of Menopur treatment. Depending on the progress, your doctor will decide to discontinue treatment with Menopur and not administer the hCG injection. In this case, you should use a contraceptive method (condom) or not have sexual intercourse until the next period begins.
Infertility in men:
Initially, 1,000 to 3,000 IU of human chorionic gonadotropin are administered, 3 times a week, until a normal serum testosterone level is reached.
Then, a dose of Menopur of 75-150 IU (1-2 vials) is administered 3 times a week, intramuscularly (IM), for several months.
Use in children
Menopur is not indicated for use in children.
INSTRUCTIONS FOR USE:
If your doctor tells you to inject Menopur yourself, you should follow any instructions that they
provide.
The first injection of this medicine should be administered under the supervision of a doctor or nurse.
Menopur is provided as a powder in a vial and should be dissolved with a syringe of solvent before injection. The liquid to be used to dilute Menopur is provided in a pre-filled syringe.
Menopur 600 IU should be reconstituted with a pre-filled syringe of solvent before use.
After dissolving the powder with the solvent, the vial contains medication for several days of treatment, so you should be sure to only withdraw the amount of medication that your doctor has prescribed.
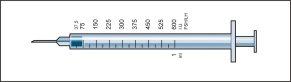
Your doctor has prescribed a dose of Menopur in IU (units). You should use one of the 9 administration syringes graduated in IU (units) FSH/LH provided.
Follow these steps:
|
|
|
|
|
|
1 | 2 | 3 | 4 |
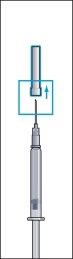
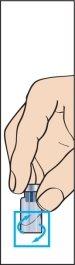
- Remove the protective cap from the powder vial and the rubber stopper from the pre-filled syringe of solvent (image 1).
- Firmly attach the long, thin needle (reconstitution needle) to the pre-filled syringe of solvent and remove the protective cap (image 2).
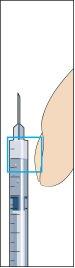
- Insert the needle vertically through the center of the rubber stopper of the powder vial and slowly inject all of the liquid to avoid forming air bubbles (image 3).
4 When the solvent is added, a slight overpressure is created in the vial. Therefore, release the plunger of the syringe so that it rises by itself for about 10 seconds. This will eliminate the excess pressure in the vial (image 4).
Remove the syringe and reconstitution needle.
|
|
|
|
|
5 | 6 | 7 | 8 |
- The powder will dissolve quickly (in 2 minutes) to form a clear solution. Although this usually happens when only a few drops of solvent have been added, the total amount of solvent should be added. To help the powder dissolve, gently move the vial (image 5). Do not shake, as this can cause air bubbles to form.
Do not usethe reconstituted solution if it contains particles or is not clear.
The powder vial is now dissolved with a pre-filled syringe of solvent and ready to use.
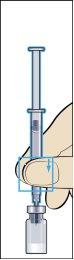
- Take the administration syringe with the pre-attached needle and insert the needle vertically into the center of the vial. The administration syringe already contains a small amount of air that should be injected into the vial above the liquid. Hold the vial upside down and withdraw the prescribed dose of Menopur into the administration syringe for injection (image 6).
REMEMBER: since the vial contains medication for several days of treatment, you should ensure that you only withdraw the amount of medication that your doctor has prescribed.
If you have been prescribed Bravelle at the same time as Menopur, you can mix the two medicines by reconstituting Menopur and injecting the prescribed dose of Menopur into the reconstituted Bravelle solution. Withdraw the mixed solution, so you can inject both medicines together and not separately.
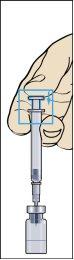
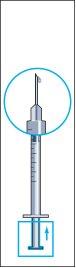
- Remove the syringe from the vial and withdraw a small amount of air from the syringe (image 7).
- Gently tap the administration syringe so that all air bubbles remain at the top (image 8). Carefully push all the air until the first drop of liquid from the solution comes out of the needle.
Your doctor or nurse will tell you where to inject (e.g. in the front of the thigh, abdomen, etc.).
Before injection, clean the skin at the injection site with an alcohol swab.
|
9 |
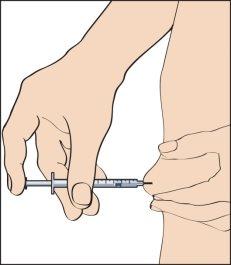
- To inject, pinch the skin to produce a fold, and insert the needle in a rapid motion at 90 degrees to the body. Press the plunger to inject the solution (image 9), and then remove the syringe.
After removing the syringe, apply pressure to the injection site to contain any bleeding. Gentle massage at the injection site will help to disperse the solution under the skin.
Do not throw away the used material in the trash, it should be disposed of properly.
- For subsequent injections with the reconstituted Menopur solution, repeat steps 6 to 9.
If you use more Menopur than you should.In case of overdose or if the solution is swallowed accidentally, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Menopur,do not use a double dose to make up for the forgotten doses. Please tell your doctor or pharmacist.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, Menopur can cause adverse effects, although not all people suffer from them.
Treatment with Menopur may cause high levels of activity in the ovariesthat can lead to a disease called Ovarian Hyperstimulation Syndrome (OHSS), especially in women with polycystic ovaries.Symptoms include: distension and discomfort in the abdomen, nausea, vomiting, diarrhea, weight gain.In some severe cases of OHSS, rare complications have been reported, such as fluid accumulation in the abdomen, pelvis, and/or pleural cavity, difficulty breathing, and decreased urine production.
Formation of blood clots in blood vessels (thromboembolism) and ovarian torsion. If you experience any of these symptoms, contact your doctor immediately, even if they develop a few days after the last injection.
Allergic reactions (hypersensitivity) may occur when using this medication. Symptoms of these reactions may include: rash, itching, swelling of the throat, and difficulty breathing.If you experience any of these symptoms, contact your doctor immediately.
The following frequent adverse effectsaffect between 1 and 10 out of 100 treated patients:
- Abdominal pain
- Headache
- Nausea
- Abdominal bloating (fullness)
- Pelvic pain
- Hyperstimulation of the ovaries resulting in high levels of activity (Ovarian Hyperstimulation Syndrome)
- Local reactions at the injection site (such as pain, redness, bruising, swelling, and/or irritation).
The following infrequent adverse effectsaffect between 1 and 10 out of 1,000 treated patients:
- Vomiting
- Abdominal discomfort
- Diarrhea
- Fatigue
- Dizziness
- Fluid-filled sacs within the ovaries (ovarian cyst)
- Breast complications (including chest pain, breast tension, breast discomfort, nipple pain, and breast swelling)
- Hot flashes
The following rare adverse effectsaffect between 1 and 10 out of 10,000 treated patients:
- Acne
- Skin rash
In addition to the adverse effects indicated above, the following adverse effects have been reported after marketing of Menopurwith an unknown frequency:
- Visual disturbances
- Fever
- Feeling unwell
- Allergic reactions
- Weight gain
- Muscle and joint pain (e.g., back, neck, arm, and leg pain)
- Ovarian torsion as a complication of increased ovarian activity due to hyperstimulation.
- Itching
- Hives
- Blood clots as a complication of increased ovarian activity due to hyperstimulation.
Reporting of adverse effects.
If you experience any adverse effects, consult your doctor or pharmacist, even if they are possible adverse effects not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines https://www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Menopur
Keep out of sight and reach of children.
Before reconstitution, store in the refrigerator (2°C – 8°C). Do not freeze. Keep in the original package to protect from light.
After reconstitution, the solution can be stored for a maximum of 28 days at no more than 25°C.
The reconstituted solution should not be administered if it contains particles or is not transparent.
Do not use Menopur after the expiration date stated on the packaging after "CAD". The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and unused medicines at the SIGRE collection point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Menopur
The active ingredient is highly purified menotropin (human menopausal gonadotropin, hMG-HP) corresponding to 600 IU of FSH with follicle-stimulating hormone activity and 600 IU of LH with luteinizing hormone activity.
After reconstitution, 1 ml of the reconstituted solution contains 600 IU of highly purified menotropin.
The other components of the powder are: lactose monohydrate, polysorbate 20, disodium phosphate heptahydrate (as a buffering agent and pH adjuster), and phosphoric acid (for pH adjustment).
The components of the solvent are: metacresol and water for injection.
Appearance of the Product and Package Contents.
MENOPUR is a powder and solvent for injectable solution.
Menopur is a lyophilized, white to grayish powder presented in a glass vial with a pre-filled syringe containing solvent, a clear, colorless solution for reconstitution, 1 reconstitution needle, and 9 graduated disposable syringes with pre-attached needles for administration.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder
Ferring, S.A.U
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
- Spain.
Manufacturer:
FERRING GmbH
Wittland 11,
D-24109 Kiel
GERMANY
This prospectus was approved inSeptember 2015
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MENOPUR 600 IU POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, -Active substance: human menopausal gonadotrophinManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.U.Prescription required
Online doctors for MENOPUR 600 IU POWDER AND SOLVENT FOR INJECTION
Discuss questions about MENOPUR 600 IU POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions