
LIMIFEN 0.5 mg/ml INJECTABLE SOLUTION

How to use LIMIFEN 0.5 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What LIMIFEN 0.5 mg/ml Solution for Injection is and what it is used for
- What you need to know before you use LIMIFEN 0.5 mg/ml Solution for Injection
- How to use LIMIFEN 0.5 mg/ml Solution for Injection
- Possible side effects
- Storage of LIMIFEN 0.5 mg/ml Solution for Injection
- Container Content and Additional Information
Introduction
Package Leaflet: Information for the User
LIMIFEN 0.5 mg/ml Solution for Injection
Alfentanil
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What LIMIFEN 0.5 mg/ml Solution for Injection is and what it is used for
- What you need to know before you use LIMIFEN 0.5 mg/ml Solution for Injection
- How to use LIMIFEN 0.5 mg/ml Solution for Injection
- Possible side effects
- Storage of LIMIFEN 0.5 mg/ml Solution for Injection
- Contents of the pack and further information
1. What LIMIFEN 0.5 mg/ml Solution for Injection is and what it is used for
What is LIMIFEN
LIMIFEN contains the active substance "alfentanil".
What LIMIFEN is used for
LIMIFEN is a medicine used for general anesthesia (making the patient sleep while undergoing an operation).
LIMIFEN is indicated for use in adults as:
Opioid analgesic together with other medicines in regional anesthesia and in short-term or long-term interventions (administering new doses at certain intervals).
LIMIFEN is indicated for use in children of all ages, including newborns, as:
Opioid in association with a hypnotic to induce anesthesia, as an opioid analgesic in association with general anesthesia, and for short-term and long-term interventions.
2. What you need to know before you use LIMIFEN 0.5 mg/ml Solution for Injection
Do not use LIMIFEN:
? if you are known to be intolerant to alfentanil or other opioids (medicines with a similar effect to morphine)
Be careful with LIMIFEN:
Tell your doctor if you have:
? Lung diseases or breathing problems
? Brain diseases
? Thyroid problems
? Liver or kidney problems
- You or any member of your family have abused alcohol or experienced dependence on it, prescription medicines, or illegal drugs ("addiction").
- You smoke.
- You have had mood problems (depression, anxiety, or personality disorder) or have been treated by a psychiatrist for other mental illnesses.
This medicine contains alfentanil, which is an opioid. Repeated use of opioid analgesics can make the drug less effective (the body gets used to it). It can also cause dependence and addiction, which can lead to a potentially fatal overdose. It is essential that you consult your doctor if you are concerned about becoming dependent on LIMIFEN.
LIMIFEN is a medicine used for general anesthesia and should only be used by qualified and medically skilled personnel.
Tell your doctor if you are pregnant, may become pregnant, or are breastfeeding (see the Pregnancy section for more information).
Tell your doctor or pharmacist if you have experienced increased sensitivity to pain despite taking increasing doses (hyperalgesia). Your doctor will decide if you need to change the dose or stop taking this medicine.
It is essential to keep in mind that LIMIFEN can weaken breathing. This can also occur after the operation. Therefore, you should be monitored for a period after the operation. Consult your doctor or nurse immediately if you experience intense drowsiness or breathing problems.
Using LIMIFEN with food and drinks
Do not drink alcohol when you are going to use LIMIFEN, as the effects of this medicine may be enhanced.
If you regularly drink alcohol, inform your doctor.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before taking any medicine.
If you are pregnant or think you may be pregnant, consult your doctor; they will decide if you can use LIMIFEN.
LIMIFEN can be excreted in breast milk; therefore, breastfeeding is not recommended until 24 hours after the last administration. Do not use breast milk that has been expressed within 24 hours following the last administration of LIMIFEN.
If women receive this drug during pregnancy, there is a risk that the newborn may experience neonatal withdrawal syndrome.
Driving and using machines
LIMIFEN may decrease your alertness or affect your ability to drive vehicles. It is recommended to wait at least 24 hours after administration of LIMIFEN before driving vehicles or using hazardous machinery.
Always ask your doctor for advice.
Information for athletes
Athletes are informed that this medicine contains a component that may result in a positive doping test.
CNS depressants, alcohol, and other medicines
Tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including those bought without a prescription or herbal remedies. Inform your doctor or pharmacist if you are taking any of the following medicines, as LIMIFEN may affect their proper functioning:
? Powerful analgesics, medicines that affect the central nervous system (CNS depressants), especially medicines known as benzodiazepines or related medicines, alcohol, and some illegal drugs. If you are taking powerful analgesics or CNS depressants (e.g., sleeping pills, tranquilizers, medicines for mental disorders, alcohol, some illegal drugs), you should inform your doctor because the dose of LIMIFEN may need to be reduced. Additionally, if you receive a powerful analgesic or another CNS depressant after receiving LIMIFEN during surgery, it may be necessary to reduce the analgesic or other CNS depressant to reduce the risk of potentially serious side effects such as breathing difficulties or shallow breathing, severe drowsiness, and decreased consciousness, coma, and death. The concomitant use of opioids and drugs used to treat epilepsy, neuropathic pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose and respiratory depression and can put the patient's life at risk.
? Erythromycin (an antibiotic), cimetidine (a medicine for stomach acid), or diltiazem (a medicine used for a certain type of heart disease). These medicines increase the effects of LIMIFEN; when such drugs have been administered, the necessary dose of LIMIFEN will be lower than usual.
? Certain anti-HIV medicines (e.g., ritonavir) or certain medicines for fungal infections (e.g., fluconazole, ketoconazole, itraconazole). It may be necessary to change the dose of LIMIFEN.
- Medicines for depression known as Monoamine Oxidase Inhibitors (MAOIs). It is not recommended to take these medicines in the two weeks prior to or at the same time as LIMIFEN is administered.
- Medicines for depression known as Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs). It is generally not recommended to take these medicines at the same time as LIMIFEN.
LIMIFEN Solution for Injection contains sodium
This medicine contains 0.15 mmol (or 3.54 mg) of sodium per milliliter of solution. If you need large amounts of the solution (e.g., more than 6.5 milliliters, corresponding to more than 1 mmol of sodium), your doctor will take this into account if you are on a sodium-controlled diet.
Children and adolescents
LIMIFEN may cause breathing difficulties and muscle stiffness, especially in babies and very young children.
When LIMIFEN is administered to babies and young children:
Their breathing should be carefully monitored during the operation and for some time after.
The doctor may administer a muscle relaxant to prevent stiffness.
LIMIFEN contains sodium
Ampoule of 2 ml of LIMIFEN: This medicine contains less than 1 mmol of sodium (23 mg) per ampoule of 2 ml, i.e., essentially "sodium-free".
Ampoule of 10 ml of LIMIFEN: This medicine contains 35.4 mg of sodium (main component of cooking/table salt) in each ampoule of 10 ml. This is equivalent to 1.8% of the maximum recommended daily sodium intake for an adult.
3. How to use LIMIFEN 0.5 mg/ml Solution for Injection
LIMIFEN is injected into a vein (intravenous use).
The amount of LIMIFEN depends on your situation. Your doctor will determine how much LIMIFEN you need based on your weight, age, and medical condition.
Use in children and adolescents
LIMIFEN is used with other medicines (anesthetics or sedatives) in children and adolescents.
When LIMIFEN is administered as an injection with other medicines such as anesthetics or pain relievers in older children, the initial dose is usually 10 to 20 micrograms per kilogram of body weight. If necessary, additional injections of 5 to 10 micrograms per kilogram of body weight may be administered.
When LIMIFEN is administered as an infusion to maintain pain relief during a surgical intervention, the usual dose is 0.5 to 2 micrograms per kilogram of body weight per minute. If the LIMIFEN infusion is combined with an anesthetic, the usual dose is approximately 1 microgram per kilogram of body weight per minute.
In babies, LIMIFEN may be administered in smaller, adjusted doses according to response.
In adolescents, LIMIFEN will be administered in doses similar to those administered in adults.
If you use more LIMIFEN than you should:
In the unlikely event that you are given an overdose, your doctor will take the necessary actions.
The main symptom is difficulty breathing.
Information for the doctor in case of overdose
Immediate measures:
In case of hypoventilation or apnea, administer oxygen and control breathing.
A specific opioid antagonist should be used to control respiratory depression. Note that additional doses may be required.
Other measures:
If muscle stiffness accompanies respiratory depression, it may be necessary to use an intravenous neuromuscular blocking agent.
If hypotension occurs or persists, it should be considered as possible hypovolemia and, therefore, parenteral fluid administration.
The patient should be carefully monitored, and if necessary, provide adequate support measures.
4. Possible side effects
Like all medicines, LIMIFEN can cause side effects, although not everybody gets them.
Very common: affects more than 1 in 10 people
Common: affects between 1 and 10 in 100 people
Uncommon: affects between 1 and 10 in 1,000 people
Rare: affects between 1 and 10 in 10,000 people
Very rare: affects less than 1 in 10,000 people
Unknown: frequency cannot be estimated from the available data
Very rare (affects less than 1 in 10,000 people):
- Hypersensitivity (allergy) including anaphylactic reaction, anaphylactoid reaction, and urticaria
- Disorientation
- Loss of consciousness (postoperative period), convulsion, myoclonus (sudden, brief, involuntary contraction of muscles)
- Miosis (pupillary constriction)
- Cardiac arrest
- Respiratory arrest, respiratory depression (slow or insufficient breathing) (including fatal outcome), cough
- Erythema (redness), rash (skin eruption)
- Pyrexia (fever)
Other side effects that may occur include:
- Abnormally slow and/or weak breathing or temporary interruption of breathing
- Spasms of the airways and larynx
- Hypo
- Slow, fast, or irregular heart rate
- Low or high blood pressure
- Muscle stiffness or involuntary muscle movements, including slow movements, stiffness, or jerks
- Dizziness
- Nausea and vomiting
- Cardiac or respiratory arrest
- Chills
- Fatigue
- Fever
- Headache
- Drowsiness
- Lack of response to stimuli
- Loss of consciousness
- Convulsions
- Agitation
- Crying
- Disorientation
- Feeling of well-being or euphoria
- Sedation
- Nasal bleeding
- Cough
- Excess carbon dioxide in the blood
- Visual disturbance
- Constricted pupils
- Allergic dermatitis
- Itching
- Excessive sweating
- Redness of the skin
- Rash
Other complications may occur, such as postoperative agitation or confusion; respiratory or neurological complications; complications due to anesthesia or endotracheal intubation. You may experience pain, including pain at the injection site, vein, or pain due to the procedure.
Rarely, serious allergic reactions may occur and may include:
- Swelling of the face, lips, mouth, tongue, or throat
- Difficulty swallowing or breathing
- Itchy skin rash (urticaria)
Possible side effects in children and adolescents
The frequency, type, and severity of side effects in children and adolescents are similar to those described above, with some exceptions. In newborns, mild or moderate muscle stiffness may occur frequently. Less frequently, there may be cases of severe stiffness and spasms accompanied by breathing difficulties.
If you think any of the side effects you are experiencing is serious, or if you notice any side effects not mentioned in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaramRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of LIMIFEN 0.5 mg/ml Solution for Injection
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do not use LIMIFEN after the expiry date stated on the packaging.
6. Container Content and Additional Information
Composition of LIMIFEN0.5 mg/ml INJECTABLE SOLUTION
-LIMIFEN is a sterile aqueous, isotonic solution without preservatives that contains alfentanil.
Each milliliter of LIMIFEN contains as an active principle 0.5 milligrams (mg) of alfentanil (as alfentanil hydrochloride).
The other components are: sodium chloride and water for injection.
Appearance of the Product and Container Content
LIMIFEN is presented in containers of 5 ampoules containing 2 milliliters or 10 milliliters.
Marketing Authorization Holder and Manufacturer
Holder:
Piramal Critical Care B.V.
Rouboslaan 32, 2252 TR
Voorschoten
Netherlands
Manufacturer: Piramal Critical Care B.V.
Rouboslaan 32, 2252 TR
Voorschoten
Holland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Euromed Pharma Spain S.L.
C/Eduard Maristany, 430-432
08918 Badalona
Barcelona - Spain
Date of the last revision of this prospectus: August 2022
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
This information is intended only for healthcare professionals
Posology and Method of Administration
LIMIFEN should be used as bolus injections (in short-duration interventions) or as supplemental boluses or by continuous infusion (in long-duration interventions).
The dose of LIMIFEN must be individualized according to age, body weight, physical condition, underlying pathological condition, use of other medications, and type of surgical intervention and anesthesia.
To avoid bradycardia, a small intravenous (IV) dose of an anticholinergic medication can be administered just before anesthetic induction.
Dose for Adults:
Use in Short Interventions and Outpatient Patients
Small doses of LIMIFEN are more useful in minor and short surgical interventions, and in outpatient patients, as long as cardiopulmonary monitoring equipment is available.
An IV bolus dose of 7 to 15 micrograms/kg (1 to 2 ml/70 kg) is normally adequate for interventions lasting less than 10 minutes. If the duration of the intervention is extended beyond 10 minutes, additional increments of 7 to 15 micrograms/kg (1 to 2 ml/70 kg) will be administered every 10 to 15 minutes or as needed.
Although ventilatory assistance should be available, in most cases spontaneous respiration should be maintained with a dose of 7 micrograms/kg (1 ml/70 kg) or less, injected slowly; with this technique, increments of 3.5 micrograms/kg (0.5 ml/70 kg) are recommended.
When post-operative nausea occurs, it is relatively short-lived and usually controllable with usual measures.
For Medium-Duration Interventions
The initial IV bolus dose should be adapted to the expected duration of the surgical intervention as indicated:
Duration of Intervention (min.) | IV Dose of LIMIFEN in Bolus | |
micrograms/kg | ml/70 kg | |
10-30 30-60 >60 | 20-40 40-80 80-150 | 3-6 6-12 12-20 |
When the surgical intervention is longer or more aggressive, analgesia can be maintained by:
- either increments of 15 micrograms/kg (2 ml/70 kg) of LIMIFEN when necessary (to avoid post-operative respiratory depression, LIMIFEN should not be administered during the last 10 minutes of surgical intervention);
- or by an infusion of LIMIFEN at a rate of 1 microgram/kg/min (0.14 ml/70 kg/min) until 5 to 10 minutes before the end of the intervention.
Painful stimulus periods can be controlled by means of additional small doses or by increasing the infusion rate.
Lower doses of LIMIFEN can be used when anesthesia is complemented with other agents.
When LIMIFEN is used without N2O/O2 or without another inhaled anesthetic, a higher maintenance dose of LIMIFEN is required.
For Longer Surgical Interventions
LIMIFEN can be used as the analgesic component of anesthesia during longer surgical interventions, especially when rapid extubation is required. By administering an individually adapted initial dose and adjusting the infusion rate to the severity of the surgical stimulus and the patient's reactions, optimal analgesia and stable autonomic nervous system function will be maintained.
Special Populations
Pediatric Population
There should be ventilatory assistance equipment available for use in children of any age, even for short-duration interventions in children with spontaneous respiration.
Data in children, particularly those from 1 month to 1 year of age, are limited (see section 5.2).
- Neonates (from 0 to 27 days): Pharmacokinetics is very variable in neonates, particularly in premature infants. Clearance and protein binding are lower, and a lower dose of LIMIFEN may be necessary. Neonates should be closely monitored, and the dose of LIMIFEN should be adjusted according to the response.
- Infants and children (from 28 days to 23 months of age): Clearance may be higher in infants and children compared to adults. It may be necessary to increase the infusion rate of LIMIFEN to maintain analgesia.
- Children (from 2 to 11 years of age): Clearance could be slightly higher in children, and an increase in infusion rate may be necessary.
- Adolescents: The pharmacokinetics of alfentanil in adolescents is similar to that in adults, and no specific dosing recommendations are required.
Dosing Recommendations for Pediatric Patients
The wide variability of response to LIMIFEN makes it difficult to provide dosing recommendations for younger children. For older children, it is considered appropriate to use a bolus dose of 10 to 20 µg/kg of LIMIFEN to induce anesthesia (e.g., to supplement propofol or inhaled anesthesia) or as an analgesic. LIMIFEN can be administered in supplemental boluses of 5 to 10 µg/kg at appropriate intervals.
To maintain anesthesia in children during the intervention, LIMIFEN can be administered with an infusion rate of 0.5 to 2 µg/kg/min. The dose should be adjusted according to the needs of each patient. When combined with an intravenous anesthetic, the recommended dose is approximately 1 µg/kg/min.
There may be a higher risk of respiratory complications and muscle rigidity when LIMIFEN is administered to neonates and younger children than when administered to older children and adults. For this reason, younger pediatric patients should be monitored immediately after administration of LIMIFEN. There should be ventilatory assistance equipment available for use in children of any age, even for short-duration interventions in children with spontaneous respiration.
If LIMIFEN is used in neonates and infants, consideration should be given to the use of a muscle relaxant due to the risk of muscle rigidity. All children should be monitored for a sufficient period after the end of treatment with LIMIFEN to ensure return to spontaneous respiration.
In neonates, a lower dose of LIMIFEN may be necessary due to pharmacokinetic variations.
Neonates should be closely monitored, and the dose of LIMIFEN should be adjusted according to the response.
Debilitated and Elderly Patients
The initial dose should be reduced in elderly patients (over 65 years of age) and in debilitated patients. The effect of the initial dose should be taken into account when determining dose supplements.
Method of Administration
LIMIFEN is administered by bolus injection, or by bolus supplements for increments, or by infusion.
Special Warnings and Precautions for Use
As with all potent opioids, the following may occur:
Respiratory Depression
Respiratory depression is dose-dependent and can be reversed with specific opioid antagonists, but additional doses of these may be necessary since respiratory depression may last longer than the action of the opioid antagonist. Deep analgesia is accompanied by significant respiratory depression and loss of consciousness that may persist or recur during the post-operative period. Therefore, patients who receive LIMIFEN should remain under adequate surveillance. Resuscitation equipment and opioid antagonists should be available. Hyperventilation during anesthesia may alter the patient's response to CO2, affecting respiration in the post-operative period.
Risk Due to Concomitant Use of Central Nervous System (CNS) Depressants, Especially Benzodiazepines or Related Medications.
In patients with spontaneous respiration, the concomitant use of LIMIFEN and CNS depressants, especially benzodiazepines or related medications, may increase the risk of deep sedation, respiratory depression, coma, and death. If it is decided to administer LIMIFEN concomitantly with a CNS depressant, especially a benzodiazepine or a related medication, the effective lowest dose of both medications should be administered for the shortest concomitant use period. Patients should be closely monitored for signs and symptoms of respiratory depression and deep sedation. In this sense, it is strongly recommended to inform patients and their caregivers to recognize these symptoms (see Interactions).
Muscle Rigidity
Muscle rigidity can be induced, which could also include thoracic muscles, but this can be avoided by taking the following measures: slow IV injection (generally sufficient for low doses), premedication with benzodiazepines, and use of muscle relaxants. Non-epileptic (myo)clonic movements may occur.
Cardiac Disease
If anticholinergic medications are administered in insufficient quantities or if non-vagolytic muscle relaxants are used, bradycardia and even asystole may be observed. Bradycardia can be treated with atropine.
Special Dosage Conditions
As with other opioids, LIMIFEN may induce hypotension, especially in hypovolemic patients. Adequate measures should be taken to maintain stable blood pressure.
Alfentanil is metabolized mainly by the cytochrome P450 3A4 enzyme pathway.
Available pharmacokinetic data indicate that the metabolism of alfentanil may be inhibited by some medications (see Interactions with Other Medications and Other Forms of Interaction). This could increase the risk of delayed or prolonged respiratory depression, so concomitant use of these medications requires careful patient monitoring, and it may be necessary to reduce the dose of LIMIFEN.
The use of rapid bolus injections of opioids should be avoided in patients with compromised intracerebral compliance; in these patients, the transient decrease in mean arterial pressure may be accompanied, on occasion, by a short-term reduction in cerebral perfusion pressure.
Patients undergoing chronic opioid treatment or with a history of opioid dependence may require higher doses.
It is recommended to reduce the dosage in debilitated and elderly patients. As with other opioids, LIMIFEN should be administered with caution in the following conditions: uncontrolled hypothyroidism, pulmonary diseases, decreased respiratory reserves, alcoholism, and altered hepatic or renal function. These patients also require prolonged post-operative monitoring.
Pediatric Population
There may be a higher risk of respiratory complications and muscle rigidity when LIMIFEN is administered to neonates and younger children than when administered to older children and adults. For this reason, younger pediatric patients should be monitored immediately after administration of LIMIFEN. There should be ventilatory assistance equipment available for use in children of any age, even for short-duration interventions in children with spontaneous respiration.
If LIMIFEN is used in neonates and infants, consideration should be given to the use of a muscle relaxant due to the risk of muscle rigidity. All children should be monitored for a sufficient period after the end of treatment with LIMIFEN to ensure return to spontaneous respiration.
In neonates, a lower dose of LIMIFEN may be necessary due to pharmacokinetic variations.
Neonates should be closely monitored, and the dose of LIMIFEN should be adjusted according to the response.
Instructions for Correct Administration of the Preparation
If desired, LIMIFEN can be mixed with intravenous infusions of sodium chloride or glucose. Such dilutions are compatible with plastic infusion sets. These should be used within 24 hours after preparation.
Keep this medication out of sight and reach of children.
Use gloves while opening and/or handling the ampoule.
Accidental skin exposure should be treated by rinsing the affected area with water. Avoid using soap, alcohol, or other cleaning materials that may cause chemical or physical abrasions to the skin.
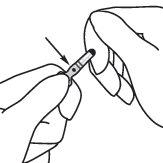
- Hold the ampoule between the index and thumb fingers, leaving the tip of the ampoule free.
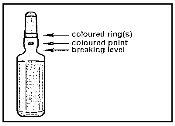
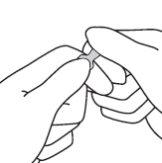
- With the other hand, hold the tip of the ampoule by placing the index finger against the neck of the ampoule and the thumb on the colored point parallel to the colored identification rings.
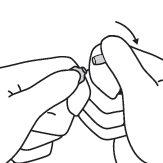
- Maintaining the thumb in that position, break the tip of the ampoule while firmly holding the other part of the ampoule in the hand.


- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIMIFEN 0.5 mg/ml INJECTABLE SOLUTIONDosage form: SUBLINGUAL TABLET, 30 MICROGRAMSActive substance: sufentanilManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, 0.0785 mg/mlActive substance: fentanylManufacturer: Kern Pharma S.L.Prescription requiredDosage form: INJECTABLE, 50 microgramsActive substance: fentanylManufacturer: Laboratorios Basi Industria Farmaceutica S.A.Prescription required
Online doctors for LIMIFEN 0.5 mg/ml INJECTABLE SOLUTION
Discuss questions about LIMIFEN 0.5 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















