
LATANOPROST/TIMOLOL STADA 50 micrograms/ml / 5 mg/ml EYE DROPS SOLUTION

How to use LATANOPROST/TIMOLOL STADA 50 micrograms/ml / 5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Latanoprost/Timolol STADA 50 micrograms/ml + 5 mg/ml eye drops, solution
Latanoprost/Timolol
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Latanoprost/Timolol Stada and what is it used for
- What you need to know before you use Latanoprost/Timolol Stada
- How to use Latanoprost/Timolol Stada
- Possible side effects
- Storage of Latanoprost/Timolol Stada
- Contents of the pack and other information
1. What is Latanoprost/Timolol Stada and what is it used for
Latanoprost/Timolol Stada is a medicine used for the treatment of increased intraocular pressure(pressure in the eye).
Latanoprost/timolol is a combination of two active substances: latanoprost (a prostaglandin derivative) and timolol maleate (a beta-blocker).
Inside the eye, there is a fluid called aqueous humor. This fluid is drained back into the bloodstream, which maintains the necessary pressure inside the eye. If this outflow is blocked, the pressure inside the eye increases.
Among other things, beta-blockers reduce the pressure inside the eye by reducing the production of aqueous humor. Prostaglandins promote the outflow of aqueous humor.
Latanoprost/timolol Stada is used:
- To reduce the pressure inside the eye in patients with open-angle glaucoma (nerve damage caused by excessive pressure inside the eye).
- To reduce the pressure inside the eye in patients where the effect of beta-blockers or prostaglandin derivatives alone is not sufficient.
2. What you need to know before you use Latanoprost/Timolol Stada
Do notuse Latanoprost/Timolol Stada eye drops, solution
 if you are allergicto latanoprost or timolol, beta-blockers or any of the other ingredients of latanoprost/timolol (described in section 6).
if you are allergicto latanoprost or timolol, beta-blockers or any of the other ingredients of latanoprost/timolol (described in section 6).- if you have or have had respiratory problems such as asthma, severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing and/or prolonged coughing).
- if you have severe heart problems or rhythm disorders.
- if you are pregnant (or trying to become pregnant)
- if you are breast-feeding.
Warnings and precautions
Before using this medicine, tell your doctor if you have or have had
- coronary heart disease (symptoms may include chest pain or feeling of oppression, shortness of breath or choking), heart failure, low blood pressure
- heart rhythm disorders, such as slow heartbeat
- respiratory problems, asthma or chronic obstructive pulmonary disease
- circulatory failure (such as Raynaud's disease or Raynaud's syndrome)
- diabetes as timolol may mask the signs and symptoms of hypoglycemia
- hyperthyroidism as timolol may mask the signs and symptoms
- any type of eye surgery (including cataract surgery)
- eye problems (such as eye pain, eye irritation, eye inflammation or blurred vision)
- dry eye (dry eye syndrome)
- angina (in particular a type known as Prinzmetal's angina)
- severe adverse reactions that usually require hospital treatment
- if you have had or are having a viral eye infection caused by the herpes simplex virus (HSV)
Use of contact lenses: You can continue using latanoprost/timolol but follow the instructions for contact lens users in the section “Latanoprost/Timolol Stada contains benzalkonium chloride”.
Before undergoing surgery, inform your doctor that you are using latanoprost/timolol as latanoprost/timolol may affect the effects of some medicines during anesthesia.
Use in athletes
This medicine contains timolol which may produce a positive result in doping tests.
Use of Latanoprost/Timolol Stada with other medicines
Latanoprost/timolol may affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Inform your doctor if you are using or plan to use medicines to reduce blood pressure, heart medicines or to treat diabetes.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines (including the use of eye drops).
 Medicines can influence each other and interactions may occur. You should take this into account if you are taking or using any of the following types of medicines:
Medicines can influence each other and interactions may occur. You should take this into account if you are taking or using any of the following types of medicines:
- Calcium antagonists(e.g. for coronary heart disease or high blood pressure) Guanethidine(for high blood pressure)
Beta-blockers(for high blood pressure)
Antiarrhythmics(medicines to normalize heart rhythm)
Digitalis glycosides(for heart failure)
Parasympathomimetic agents(e.g. for the treatment of glaucoma)
Taking/using latanoprost/timolol with the above medicines may cause low blood pressure and/or decrease heart rate.
- Medicines that act similarly to latanoprost/timolol
If used at the same time as latanoprost/timolol, the effect of other medicines with a similar action to latanoprost/timolol may be increased. For this reason, the ophthalmic use (i.e. in the eye) of two beta-blockers or two prostaglandin derivatives is not recommended.
- Clonidine
If you are using the active substance clonidine to reduce intraocular pressure along with latanoprost/timolol and you suddenly stop using clonidine, your blood pressure may increase. If you are also using beta-blockers to lower your blood pressure, your blood pressure may increase even more due to this adverse effect.
- Quinidine(used to treat heart diseases and some types of malaria)
- Antidepressantsknown as fluoxetine and paroxetine
Children and adolescents
Latanoprost/timolol is not recommended for children or adolescents.
Elderly patients
Latanoprost/timolol is also suitable for the treatment of elderly patients.
Pregnancy and breast-feeding
Pregnancy
Do not use latanoprost/timolol if you are pregnant unless your doctor considers it necessary. Inform your doctor immediately if you are pregnant, think you may be pregnant or are planning to become pregnant.
Breast-feeding
Do not use latanoprost/timolol if you are breast-feeding. Timolol and latanoprost may pass into breast milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
After administering the latanoprost/timolol eye drops, your vision may be temporarily altered.
If you experience blurred vision – mainly after putting in latanoprost/timolol eye drops – you should not:
- drive any vehicle
 use tools or machines
use tools or machines
Latanoprost/Timolol Stada contains benzalkonium chloride and phosphates
This medicine contains 0.2 mg of benzalkonium chloride in each ml. Benzalkonium chloride can be absorbed by soft contact lenses and may alter the color of the contact lenses. Remove the contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (diseases of the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching or pain in the eye after using this medicine.
This medicine contains 6.31 mg of phosphates in each ml. If you have severe damage to the transparent layer on the front of the eye (cornea), treatment with phosphates, in very rare cases, may cause cloudy patches on the cornea due to calcium.
3. How to use Latanoprost/Timolol Stada
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
Unless your doctor has prescribed otherwise, the usual doseis:
Adults, including elderly patients: insert one drop into each affected eye, once a day.
If you are using other eye drops in addition to latanoprost/timolol, these should be administered with an interval of at least five minutes.
Instructions for use
- Wash your hands and sit or stand comfortably.
- Remove the outer protective cap from the bottle.
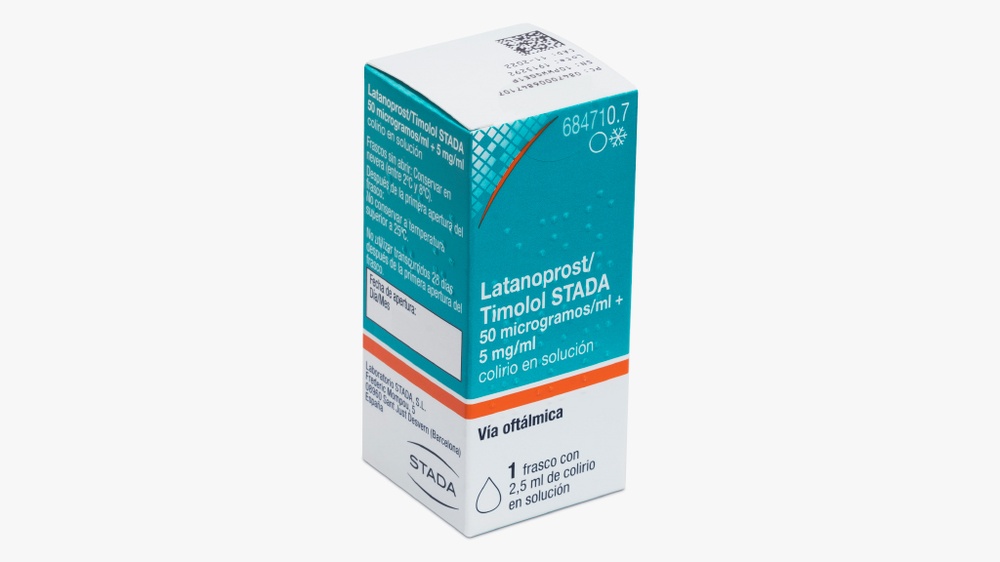
- Use the tip of your finger to gently pull the lower eyelid of the affected eye down.
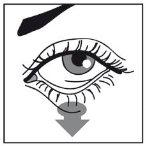
- Place the tip of the bottle close to, but not touching the eye. Gently press the bottle until one drop falls into the eye. Please make sure not to squeeze the bottle too hard, so that only one drop falls into the affected eye.
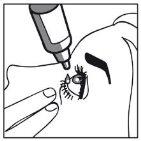
- Release the eyelid.
- After using latanoprost/timolol, press a finger against the eye, at the corner of the nose, for 2 minutes.
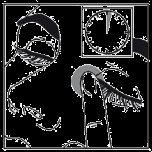

This will help latanoprost/timolol not to pass into the rest of the body
Repeat the procedure in the other eye, if your doctor has told you to do so. If the drop falls outside the eye, apply another drop.
- Close the bottle.
If you use more Latanoprost/Timolol Stada than you should
If too many drops have entered the eye, irritation and rednessmay occur.
Tell your doctor immediatelyif you or anyone else has swallowedthe drops by mistake, or if you have been using the drops more frequently than prescribed.
Keep the package of this medicine with you, so that the doctor can have more information about the medicine. He/She will decide what to do.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested. It is recommended to take the package and package leaflet of the medicine to the healthcare professional.
If you forget to use Latanoprost/Timolol Stada
If you have forgotten to use the eye drops, continue the treatment by administering the next dose in the usual way. The dose should not exceed one drop once a day in the affected eye.
Do nottake a double doseto make up for forgotten doses.
If you stop using Latanoprost/Timolol Stada
Do not stop or discontinue treatment with latanoprost/timolol without consulting your doctor first.
If you do not use latanoprost/timolol regularly or if you often forget to administer it, the success of your treatment may be at risk.
Increased intraocular pressure (pressure inside the eye) can damage the optic nerve and worsen your vision. Blindness may occur. Normally, increased intraocular pressure can hardly be perceived. The disorder can only be diagnosed through an examination performed by an ophthalmologist. If you have high intraocular pressure, regular eye exams, along with measurements of intraocular pressure, are necessary. The pressure inside the eye should be measured at least every 3 months. Once a year, visual field measurements and optic nerve exams should be performed.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
You can continue using the eye drops as usual, unless the adverse effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using latanoprost/timolol without consulting your doctor.
The following describes the side effects of using an eye drop that contains the active substances latanoprost and timolol. The most important side effect is the possibility of a gradual, permanent change in eye color. It is also possible that eye drops containing latanoprost and timolol as active ingredients may cause significant changes in heart function. If you notice changes in heart rate or cardiac function, you should talk to a doctor and tell them that you have been using latanoprost/timolol.
The frequency of the possible adverse effects described below is defined using the following convention:
Very Common(may affect more than 1 in 10 people):
- Gradual change in eye color by increasing the amount of brown pigment in the colored part of the eye called the iris. If your eye color is a mix of colors (blue-brown, gray-brown, yellow-brown, or green-brown), the change may be more noticeable than if you have eyes of a single color (blue, gray, green, or brown eyes). Any change in eye color may take years to develop. The color change may be permanent and may be more noticeable if latanoprost/timolol is used in one eye only. It does not appear to cause problems related to the change in eye color. The change in eye color does not continue after stopping treatment with latanoprost/timolol.
Common(may affect up to 1 in 10 people):
- Eye irritation (burning sensation, grittiness, itching, stinging, or feeling of a foreign body in the eye) and eye pain.
Uncommon(may affect up to 1 in 100 people):
- Headache.
- Redness of the eye, eye infection (conjunctivitis), blurred vision, tearing, eyelid inflammation, irritation, or changes in the surface of the eye.
- Skin rash or itching (pruritus).
Other Adverse Effects
The following adverse effects have been observed with latanoprost:
- Infections and infestations: Development of a viral eye infection caused by the herpes simplex virus (HSV).
Immune system disorders:
- Symptoms of an allergic reaction (swelling and redness of the skin and rash).
Nervous system disorders:
- Dizziness.
 Eye disorders:
Eye disorders:
- Changes in eyelashes and fine hair around the eye (increased number, length, thickness, and darkness), changes in the direction of eyelash growth, inflammation around the eye, swelling of the colored part of the eye (iritis/uveitis), swelling in the back of the eye (macular edema), inflammation or irritation of the eye surface (keratitis), dry eyes, fluid-filled cyst in the colored part of the eye (iris cyst), sensitivity to light (photophobia), sunken eyes (increased depth of the eyelid sulcus), eye disorder that affects the cornea characterized by lesions in the corneal epithelium with a punctate pattern (punctate epithelial erosion), swelling and fluid retention in the cornea (corneal edema), and corneal erosion (damage to the outer layer of the eyeball).
Cardiac disorders:
- Angina, worsening of angina in patients who already have heart disease, awareness of heart rhythm (palpitations).
Respiratory disorders:
- Asthma, worsening of asthma, difficulty breathing.
Gastrointestinal disorders:
- Nausea, vomiting (uncommon).
Skin disorders:
- Darkening of the skin around the eyes.
Musculoskeletal disorders:
- Joint pain, muscle pain.
General disorders:
- Chest pain.
Like other eye medicines, latanoprost/timolol is absorbed into the bloodstream. The timolol portion of this combination may cause side effects similar to those that occur with "intravenous" and/or "oral" beta-blockers. The incidence of side effects after ocular administration is lower than when medicines are taken orally or injected. The side effects described include reactions observed when beta-blockers are used to treat eye diseases:
- Generalized allergic reactions, including swelling under the skin that can occur in areas such as the face and limbs, and can obstruct the airways, causing difficulty swallowing or breathing. Urticaria or rash with itching, localized and generalized rash, itching, severe and sudden allergic reaction.
- Low blood sugar levels.
- Difficulty sleeping (insomnia), depression, nightmares, memory loss, hallucinations.
- Fainting, stroke, reduced blood supply to the brain, increased signs and symptoms of myasthenia gravis (muscle disease), dizziness, unusual sensations such as tingling, and headache.
- Signs and symptoms of eye irritation (e.g., burning, stinging, itching, tearing, redness), eyelid inflammation, corneal inflammation, blurred vision, and detachment of the layer under the retina that contains blood vessels after filtration surgery, which can cause visual disturbances, decreased corneal sensitivity, dry eye, corneal erosion (damage to the outer layer of the eyeball), and drooping of the upper eyelid (causing the eye to be half-closed), double vision.
- Ringing/buzzing in the ears (tinnitus).
 Slow heart rate, chest pain, palpitations, edema (fluid accumulation), changes in heart rate or speed, congestive heart failure (heart disease, with difficulty breathing and swelling of the feet and legs due to fluid accumulation), a type of heart rhythm disorder, heart attack, heart failure.
Slow heart rate, chest pain, palpitations, edema (fluid accumulation), changes in heart rate or speed, congestive heart failure (heart disease, with difficulty breathing and swelling of the feet and legs due to fluid accumulation), a type of heart rhythm disorder, heart attack, heart failure.
- Low blood pressure, Raynaud's phenomenon, cold hands and feet.
- Constriction of airways in the lungs (mainly in patients with pre-existing diseases), difficulty breathing, cough.
- Taste disturbances, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting.
- Hair loss, skin eruptions with a white, silvery appearance (psoriasiform rash) or worsening of psoriasis, skin rash.
- Muscle pain not caused by exercise.
- Sexual dysfunction, decreased libido.
- Muscle weakness/fatigue.
In very rare cases, some patients with severe damage to the front, transparent part of the eye (cornea) have developed cloudy areas in the cornea due to calcium deposits produced during treatment.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Latanoprost/Timolol Stada
Keep out of sight and reach of children.
Do not use this medicine after the expiration datestated on the packaging after CAD. The expiration date is the last day of the month indicated.
Please note the following storage instructions:
Unopened bottles: Store in a refrigerator between 2°C - 8°C.
After opening the bottle: Do not store above 25°C.
Once opened, the bottle - with the remaining contents - must be discarded after 4 weeks.
Otherwise, there is a risk of eye infection.
Medicines should not be disposed of through sewersor waste. Deposit the packaging and medicines you no longer need at the SIGRE collection point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Latanoprost/Timolol Stada
- The active ingredientsare: latanoprost and timolol maleate.
1 ml of eye drops contains 50 micrograms of latanoprost and 6.8 mg of timolol maleate, equivalent to 5.0 mg of timolol.
 The other ingredientsare:
The other ingredientsare:
Sodium chloride, benzalkonium chloride, sodium dihydrogen phosphate dihydrate, disodium hydrogen phosphate dodecahydrate, purified water, sodium hydroxide to adjust the pH, hydrochloric acid to adjust the pH.
Appearance of the Product and Package Contents
Latanoprost/Timolol Stada is a clear, colorless liquid, free of visible particles, packaged in a transparent dropper bottle with a screw cap.
Latanoprost/Timolol Stada is available in the following package sizes:
1 bottle containing 2.5 ml of eye drops in solution.
3 bottles, each containing 2.5 ml of eye drops in solution.
6 bottles, each containing 2.5 ml of eye drops in solution.
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Spain
Manufacturer
ROMPHARM COMPANY S.R.L.
Eroilor Street, no. 1A
Otopeni 075100, Ilfov district
Romania
or
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
 Germany
Germany
or
STADA Arzneimittel GmbH
Muthgasse 36
1190 Wien
Austria
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark: | Latanostad Comp |
Austria: | Latanoprost/Timolol Stada 50 micrograms/ml + 5 mg/ml eye drops |
Spain: | Latanoprost/Timolol Stada 50 micrograms/ml + 5 mg/ml eye drops in solution |
Finland: | Oftastad comp |
Portugal: | Latanoprost + Timolol Ciclum |
Romania: | Latanoprost/Timolol Stada HEMOFARM 50 micrograms/ml + 5 mg/ml, eye drops |
Slovak | oftalmice, solution |
Republic | |
Slovakia: | LATIMOSTAD |
The last revision of this leaflet was in:June 2022
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.09 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LATANOPROST/TIMOLOL STADA 50 micrograms/ml / 5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for LATANOPROST/TIMOLOL STADA 50 micrograms/ml / 5 mg/ml EYE DROPS SOLUTION
Discuss questions about LATANOPROST/TIMOLOL STADA 50 micrograms/ml / 5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















