KYNMOBI 25 mg SUBLINGUAL FILM-COATED TABLETS

How to use KYNMOBI 25 mg SUBLINGUAL FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kynmobi 10 mg sublingual film
Kynmobi 15 mg sublingual film
Kynmobi 20 mg sublingual film
Kynmobi 25 mg sublingual film
Kynmobi 30 mg sublingual film
apomorphine hydrochloride
Kynmobi 10 mg + 15 mg + 20 mg + 25 mg + 30 mg sublingual film
apomorphine hydrochloride
Starter Pack
Read this package leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Kynmobi and what is it used for
- What you need to know before you take Kynmobi
- How to take Kynmobi
- Possible side effects
- Storage of Kynmobi
- Contents of the pack and other information
Step-by-step instructions
1. What is Kynmobi and what is it used for
Kynmobi is a medicine that is placed under the tongue (sublingual film), which contains the active substance apomorphine hydrochloride. It is used, as needed, with other medicines for Parkinson's disease taken by mouth (orally), to reduce the time in 'OFF': a period during the day when your symptoms of the disease worsen significantly. Parkinson's disease is a progressive disease of the nervous system, which causes tremors and affects movement.
2. What you need to know before you take Kynmobi
Do not take Kynmobi
- if you are allergic to apomorphine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you are taking certain medicines for nausea called 5HT3 antagonists, including granisetron, dolasetron, palonosetron, and alosetron.
- if you are taking ondansetron (a medicine for nausea and vomiting).
- if you have a psychotic disorder, a medical term that describes many mental illnesses that cause abnormal thoughts and perceptions; people with psychosis lose contact with reality.
- if you have dementia.
- if you have aphthous ulcers: small, flat sores that appear on the soft tissues of the mouth and can cause discomfort when eating and speaking.
- if you have liver problems.
- if you have difficulty breathing.
Warnings and precautions
Talk to your doctor or pharmacist before you start taking Kynmobi:
- if you have lung problems.
- if you have heart problems.
- if you have liver problems.
- if you frequently experience nausea (feeling of dizziness) or vomiting.
- if you have low blood pressure or feel like you are going to faint or feel dizzy when you stand up. After taking Kynmobi, get up slowly if you are sitting or lying down.
- if you are taking any medicine for high blood pressure.
- if you or a family member have an abnormality in the electrocardiogram (ECG) called 'long QT syndrome'.
- if you have a mental illness with symptoms such as hallucinations, delusions, disordered thoughts, loss of contact with reality.
Mouth irritation (oral) is a very common side effect of Kynmobi. Tell your doctor if you develop any of the following signs or symptoms:
- redness,
- sores in the mouth (ulceration),
- dryness in the mouth, lips, or tongue,
- swelling,
- pain in the mouth or pain when swallowing.
These signs and symptoms disappear when treatment with Kynmobi is interrupted.
Tell your doctor if you or your family/caregiver notice that you are developing impulses or desires to behave in an unusual way and cannot resist the impulse, drive, or temptation to carry out certain activities that could harm you or others. These behaviors are called 'impulse control disorders' and may include: gambling addiction, overeating or excessive spending, abnormally high sexual desire, or an increase in sexual thoughts or feelings. This type of behavior has been reported in patients taking other medicines for Parkinson's disease. Your doctor may need to review your treatments.
Some patients develop symptoms similar to those of addiction, which lead to a compulsive desire to take high doses of Kynmobi and other medicines used to treat Parkinson's disease.
Kynmobi may cause neuroleptic malignant syndrome (a disorder of the nervous system that is usually caused by antipsychotics). This is a disorder that causes high fever, confusion, changes in breathing and heart rate, and muscle stiffness.
When you reduce your dose of Kynmobi or stop treatment, you may experience withdrawal symptoms such as: lack of interest, anxiety, depression, fatigue, sweating, panic attacks, insomnia, irritability, and pain.
This medicine may cause erections of the penis. If these evolve into prolonged painful erections, consult your doctor.
Children and adolescents
This medicine should not be used in children and adolescents under 18 years of age.
Other medicines and Kynmobi
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking:
- Ondansetron (a medicine for nausea and vomiting), as this may result in a severe drop in blood pressure and loss of consciousness.
- Domperidone, a medicine to prevent nausea.
- Medicines to lower blood pressure or treat heart disease, such as sublingual nitroglycerin. Your blood pressure may drop and cause dizziness. Lie down before and after taking sublingual nitroglycerin.
- Medicines such as clozapine to treat mental disorders.
- Certain medicines that affect heart rate.
- Medicines that may affect the levels of electrolytes (salts) in your body.
- Other medicines for Parkinson's disease.
Taking Kynmobi with alcohol
Do not drink alcohol while taking this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Kynmobi is not recommended if you are pregnant. Use an effective contraceptive method if you are of childbearing age.
It is not known whether Kynmobi is excreted in breast milk. Consult your doctor, he will indicate whether you should interrupt breastfeeding or stop Kynmobi, considering the benefit of your treatment and that of breastfeeding for your baby.
Driving and using machines
Kynmobi may cause you to feel dizzy, drowsy, or sleepy. Do not drive or operate machinery if you experience any of these side effects.
Kynmobi contains metabisulfite
Kynmobi contains metabisulfite, which can rarely cause severe hypersensitivity reactions and bronchospasm, with symptoms such as rash or itching of the skin, difficulty breathing, eyelids, face, or lips swollen, swelling or redness of the tongue. If you experience these side effects, go to the nearest hospital immediately.
This medicine contains less than 1 mmol of sodium (23 mg) per sublingual film; i.e., it is essentially 'sodium-free'.
3. How to take Kynmobi
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again.
Dose when starting treatment:
A starter pack is available, which contains 2 sublingual films of each dose. This pack is usually necessary for your doctor to find the right dose for you.
Your doctor will decide how much Kynmobi you should take and how often. You may not need all the sublingual films in the starter pack (e.g., if the correct dose for you is 20 mg, then you will not need the 25 mg and 30 mg films).
Maintenance dose:
The recommended dose of this medicine will depend on your needs and will be determined by your doctor. Do not take more than one Kynmobi film for an 'OFF' episode. You can take Kynmobi up to 5 times a day, but at least 2 hours must pass between doses.
Do not use more than 5 films per day. The maximum dose of Kynmobi per day is 150 mg.
You should place the medicine under your tongue and take it whole. Do not cut, chew, or swallow it. See the full instructions in the 'Step-by-step instructions'in this package leaflet.
If you take more Kynmobi than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken, or go to the hospital immediately. Bring the medicine pack and this package leaflet with you. This will help the doctor identify what you have taken.
If you stop taking Kynmobi
Do not stop taking Kynmobi unless your doctor tells you to, as your symptoms may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor as soon as possibleif you experience any of the following side effects:
Very common: may affect more than 1 in 10 people.
- Sleepiness.
- Yawning.
- Dizziness.
- Pain, redness, sores, or a change in sensation in the mouth, lips, or tongue.
Common: may affect up to 1 in 10 people.
- Hearing, seeing, feeling, smelling, or tasting things that do not exist.
- Abnormal, uncontrolled, involuntary movements.
- Shortness of breath.
- Oral candidiasis (thrush - a fungal infection in the mouth).
- Dizziness.
- Headache.
- Fainting.
- Blurred vision.
- A drop in blood pressure when standing up, which can cause dizziness, drowsiness, or fainting.
- Low blood pressure.
- Sudden feeling of heat.
- High blood pressure.
- Rhinorrhea.
- Vomiting.
- Swelling or dryness of the mouth, lips, tongue, or gums.
- Dry retching.
- Excessive sweating.
- Cold sweats.
- Feeling of lack of energy or fatigue.
- Malaise.
- Feeling of cold.
- Chills.
- Falls.
- Rash.
- Feeling of extreme tiredness or sleepiness.
Uncommon: may affect up to 1 in 100 people.
- Psychotic disorder: a medical term that describes many mental illnesses that cause abnormal thoughts and perceptions; people with psychosis lose contact with reality.
- Cardiac arrest.
- Irregular or slow heart rate.
- Allergic reaction. The signs can include rash, hives, itching, redness, drop in blood pressure, feeling of tightness in the throat.
- Compulsive need to take high doses of Kynmobi and other medicines used to treat Parkinson's disease.
- Recurring, unwanted thoughts, ideas, or sensations that drive you to do something repeatedly.
- Red, swollen patches on the corners of the mouth.
- Loss of appetite.
- Anxiety.
- Feeling of confusion.
- Drooling.
- Sudden episodes of sleep.
- Excessive tearing, watery eyes.
- Rapid redness of the neck, upper chest, or face.
- Pallor.
- Constipation.
- Indigestion.
- Belching.
- Difficulty or discomfort when swallowing.
- Changes in tooth color or cavities in the teeth.
- Spontaneous erection of the penis.
- Feeling of drunkenness.
- High vitamin B6.
- Agitation.
- Swelling in the legs and arms.
- Positive Coombs test.
- Hemolytic anemia, an abnormal destruction of red blood cells in the blood vessels or elsewhere in the body.
- Decrease in blood platelets, which increases the risk of bleeding or bruising.
Rare: may affect up to 1 in 1,000 people.
- Eosinophilia, an abnormally high number of white blood cells in the blood or tissues of the body.
Side effects of unknown frequency (cannot be estimated from the available data) are:
- Inability to resist the impulse to perform a dangerous action, such as gambling addiction, repetitive actions without sense, increased sexual interest, impulsive purchases or excessive spending.
- Aggression.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kynmobi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the sachets and the carton after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C. Store in the sachet to protect from light and moisture.
Keep Kynmobi in the sachet until you are ready to take it.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Composition ofKynmobi
- The active ingredient is apomorphine hydrochloride.
- The other ingredients are: disodium edetate (E385), FD&C Blue No. 1 (E133), glycerol (E422), glyceryl monostearate (E471), hydroxyethylcellulose 250 G and 250 L (E1525), hydroxypropylcellulose (E463), maltodextrin, levomenthol, pyridoxine hydrochloride (for pH adjustment), sodium hydroxide (E524) (for pH adjustment), sodium metabisulfite (E223) (see section 2), sucralose (E955), white ink (Shellac [E904], anhydrous alcohol [E1510], isopropyl alcohol, butyl alcohol, propylene glycol [E1520], concentrated ammonia solution [E527], purified water, potassium hydroxide [E525], titanium dioxide [E171]).
Appearance of the Product and Container Content
Kynmobi sublingual film is a rectangular blue-green film with a white-printed number indicating the concentration (e.g., "10" means 10 mg).
Kynmobi is available in the following package sizes:
The starter package contains 10 sublingual films with 2 sublingual films of 10 mg, 15 mg, 20 mg, 25 mg, and 30 mg each.
Other packages contain 15 or 30 sublingual films.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Bial - Portela & Cª, S.A.
À Av. da Siderurgia Nacional
4745-457 S. Mamede do Coronado
Portugal
Phone: +351 22 986 61 00
Fax: +351 22 986 61 90
e-mail: [email protected]
Manufacturer
Tesa Labtec GmbH
Heykenaukamp 10
21147 Hamburg
Germany
Local Representative
Laboratorios BIAL, S.A.
C/Alcalá 265, Edificio 2, Planta 2ª
28027 Madrid
Spain
Phone: +34 915624196
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Portugal, Italy, Germany, France: Kynmobi
Date of the last revision of this leaflet: February 2024.
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
Step-by-Step Instructions
Taking Kynmobi | ||
Step 1 | Your doctor has told you to take Kynmobi 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg. Complete steps 2 to 7to take Kynmobi. | |
Step 2 | Drink water.Before taking each Kynmobi, drink water to moisten your mouth. This helps the film dissolve more easily (see Figure A). |
Figure A |
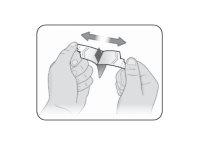
Step 3 | Open the Kynmobi packet. Hold the packet tabs between your thumb and index finger of each hand. Make sure to place your fingers just above the raised points on each tab. Gently pull the tabs to open the packet (see Figure B). |
Figure B |
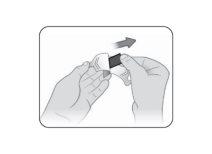
Step 4 | Remove Kynmobi from the packet. Hold Kynmobi between your fingers by the outer edges and remove it completely from the packet (see Figure C). Kynmobi should be taken whole. Discard the medication if it is broken or missing pieces. Use a new Kynmobi for the dose. |
Figure C |
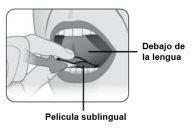
Step 5 | Place Kynmobi under your tongue. Place Kynmobi under your tongue as far back as possible (see Figure D). Close your mouth. |
Figure D |
Step 6 | Let Kynmobi dissolve completely(see Figure E).
|
Figure E |
Step 7 | Open your mouth to check if Kynmobi has dissolved completely. Kynmobi may take about 3 minutesto dissolve. When Kynmobi has dissolved completely, you can swallow. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KYNMOBI 25 mg SUBLINGUAL FILM-COATED TABLETSDosage form: INJECTABLE, 10 mg/mLActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription requiredDosage form: INJECTABLE, 10 mg/ml apomorphine hydrochlorideActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription requiredDosage form: INJECTABLE PERFUSION, 5 mg/mLActive substance: apomorphineManufacturer: Stada Arzneimittel AgPrescription required
Online doctors for KYNMOBI 25 mg SUBLINGUAL FILM-COATED TABLETS
Discuss questions about KYNMOBI 25 mg SUBLINGUAL FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions