
KABIVEN EMULSION FOR INFUSION

How to use KABIVEN EMULSION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kabiven emulsion for infusion
Read all of this leaflet carefully before you start using this medicine because it contains important information for you:
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What is Kabiven and what is it used for
- What you need to know before you use Kabiven
- How to use Kabiven
- Possible side effects
- Storage of Kabiven
- Contents of the pack and further information
-
1. What is Kabiven and what is it used for
Kabiven is presented in a three-chamber bag with an overbag. Kabiven contains the following medicines: amino acids (components used to build proteins), lipids, glucose, and electrolytes. It provides energy (in the form of sugar and lipids) and amino acids to your bloodstream when you cannot eat normally.
It is used as part of a balanced intravenous diet, together with salts, trace elements, and vitamins to fully meet your nutritional needs.
2. What you need to know before you use Kabiven
Do not use Kabiven:
- if you are allergic to any active substance or to any of the components of this medicine (listed in section 6).
- if you are allergic to products containing egg, soy, or peanut
- if you have too much fat(such as cholesterol) in your blood
- if you have severely impaired liver function
- if you are suffering from acute shock(leading to significant blood loss or allergic reaction)
- if you have bleeding disordersassociated with a known condition (such as hemophagocytic syndrome) or if your blood does not clot properly
- if you have a situation where your body has problems using proteins or amino acids
- if you have severe kidney problems
- if you have hyperglycemia (too much sugar in your blood) that requires the administration of more than 6 units of insulin per hour
- if you have high levels of electrolytes(salts) in your blood
- if you have metabolic acidosis(the levels of acid in your body fluids and tissues are too high)
- if you have too much fluidin your body (hyperhydration)
- if you have fluid in your lungs(acute pulmonary edema)
- if you are in a coma
- if you have heart problems
- if you are dehydratedwith low levels of salts
- if you have severe sepsis(a situation where your body is suffering from a severe infection)
Warnings and precautions
Tell your doctor before Kabiven is administered to you if you have:
- impaired liver function
- uncontrolled diabetes
- a situation where your body has problems using lipidsproperly
- kidney problems
- any problem with your pancreas
- thyroid problems- hypothyroidism
- sepsis(a situation where your body is suffering from an infection)
- your body has problems eliminating electrolytes
- a situation where there is not enough oxygenin your body cells
- increased serum osmolality
If during the infusion you experience fever, skin rash, chills, or difficulty breathing, inform the healthcare professional immediately. These symptoms may be caused by an allergic reaction or because you have received too much medicine (see section 4).
This medicine may affect the results of other teststhat you are given. It is important that you inform the doctor who is going to perform the tests that you are receiving Kabiven.
Your doctor may regularly perform blood tests to ensure that your body is receiving Kabiven correctly.
Children
Kabiven will not be administered to newborns or children under two years of age.
Using Kabiven with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking
- a medicine known as heparin that is used to prevent the formation of blood clots and to help dissolve them
- warfarin as vitamin K1, which is found in soybean oil and may affect the blood's ability to clot
- insulin for the treatment of diabetes
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. The safety of using Kabiven during pregnancy and breastfeeding has not been established. If parenteral nutrition is necessary during pregnancy or breastfeeding, your doctor will only administer Kabiven after careful consideration.
Driving and using machines
Kabiven is not expected to affect your ability to drive or use machines.
3. How to use Kabiven
You will receive your medicine by infusion only through a central vein. The dose of Kabiven and what size bag is used depends on your body weight in kilograms and your body's ability to use lipids and sugar. Kabiven will be infused slowly over a period of 12-24 hours. Your doctor will decide the correct dose that you or your child should receive. You may be monitored during treatment.
Children
Kabiven is not suitable for use in newborns or children under two years of age.
If you take more Kabiven than you should
It is very unlikely that you will receive more infusion than you should, as your doctor or nurse will monitor you during treatment. The effects of an overdose may include nausea, vomiting, chills, and fluid retention. Hyperglycemia (too much sugar in your blood) and electrolyte disturbances have also been reported. In the event of an overdose, there is a risk of receiving too many lipids, known as "lipid overload syndrome". For more information, see section 4 "Possible side effects". If you experience any of the symptoms described above or think you have received too much Kabiven, inform your doctor or nurse immediately. The infusion may be stopped immediately or continued at a reduced dose.
If you have any questions related to the use of this product, ask your doctor or nurse.
4. Possible side effects
Like all medicines, Kabiven can cause side effects, although not everybody gets them.
Kabiven may cause an allergic reaction (very rare, may affect up to 1 in 10,000 patients). Tell your doctor immediately if:
- a skin rash with itching appears on your body
- you have a very high temperature
- you have difficulty breathing
Other side effects include:
Common side effects (may affect up to 1 in 10 patients)
- mild increase in body temperature
Uncommon side effects (may affect up to 1 in 100 patients)
- chills
- fatigue
- stomach pain
- headache
- feeling of illness
- increase in liver enzymes. Your doctor will tell you if this happens
Very rare side effects (may affect up to 1 in 10,000 patients)
- high or low blood pressure
- difficulty breathing
- prolonged and painful erections in men
- blood problems
Lipid overload syndrome
This could happen if your body has problems using lipids and you have received too much Kabiven. It may also occur due to a sudden change in your situation (such as kidney problems or infection). Possible symptoms are fever, high levels of lipids in your blood, cells, and tissues, changes in various organs, and coma. All these symptoms will usually disappear if the infusion is stopped.
Reporting side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kabiven
Keep this medicine out of the sight and reach of children
Your doctor or the hospital pharmacist is responsible for the correct storage, use, and disposal of the Kabiven infusion. Do not store above 25°C. Do not freeze and always keep the container in the outer container.
The emulsion should not be used after the expiry date stated on the label. Any remaining emulsion should be disposed of through authorized hospital procedures.
.
6. Container Content and Additional Information
Kabiven Composition
Kabiven is available in a three-chamber bag system. Each bag contains the following different volumes depending on the four package sizes:
2566 ml | 2053 ml | 1540 ml | 1026 ml | |
Glucose (Glucose 19%) Amino acids and electrolytes (Vamin 18 Novum) Lipid emulsion (Intralipid 20%) | 1316 ml 750 ml 500 ml | 1053 ml 600 ml 400 ml | 790 ml 450 ml 300 ml | 526 ml 300 ml 200 ml |
- The active substances are
Purified soybean oil Glucose monohydrate Anhydrous glucose | 100 g 275 g 250 g | 80 g 220 g 200 g | 60 g 165 g 150 g | 40 g 110 g 100 g |
Amino Acids Alanine Arginine Aspartic acid Glutamic acid Glycine Histidine Isoleucine Leucine Lysine Methionine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine Calcium chloride 2H2O Corresponding to calcium chloride Sodium glycerophosphate (anhydrous) Magnesium sulfate 7H2O Corresponding to magnesium sulfate Potassium chloride Sodium acetate 3H2O Corresponding to sodium acetate | 12.0 g 8.5 g 2.6 g 4.2 g 5.9 g 5.1 g 4.2 g 5.9 g 6.8 g 4.2 g 5.9 g 5.1 g 3.4 g 4.2 g 1.4 g 0.17 g 5.5 g 0.74 g 0.56 g 3.8 g 2.5 g 1.2 g 4.5 g 6.1 g 3.7 g | 9.6 g 6.8 g 2.0 g 3.4 g 4.7 g 4.1 g 3.4 g 4.7 g 5.4 g 3.4 g 4.7 g 4.1 g 2.7 g 3.4 g 1.1 g 0.14 g 4.4 g 0.59 g 0.44 g 3.0 g 2.0 g 0.96 g 3.6 g 4.9 g 2.9 g | 7.2 g 5.1 g 1.5 g 2.5 g 3.6 g 3.1 g 2.5 g 3.6 g 4.1 g 2.5 g 3.6 g 3.1 g 2.0 g 2.5 g 0.86 g 0.10 g 3.3 g 0.44 g 0.33 g 2.3 g 1.5 g 0.72 g 2.7 g 3.7 g 2.2 g | 4.8 g 3.4 g 1.0 g 1.7 g 2.4 g 2.0 g 1.7 g 2.4 g 2.7 g 1.7 g 2.4 g 2.0 g 1.4 g 1.7 g 0.57 g 0.07 g 2.2 g 0.29 g 0.22 g 1.5 g 0.99 g 0.48 g 1.8 g 2.5 g 1.5 g |
- Other ingredients are
Purified egg phospholipids
Glycerol
Sodium hydroxide
Glacial acetic acid
Water for injectable preparations.
Appearance of the Product and Container Content
The glucose and amino acid solutions are clear and colorless or slightly yellow, and the lipid emulsion is white. Kabiven consists of a three-chamber bag and an overbag. An oxygen absorber is placed between the inner bag and the overbag. The inner bag is separated into three chambers by peel-type seals. The contents of the three chambers must be mixed before use, by opening the peel seals.
Package Sizes
1 x 1026 ml, 4 x 1026 ml
1 x 1540 ml, 4 x 1540 ml
1 x 2053 ml, 4 x 2053 ml
1 x 2566 ml, 3 x 2566 ml
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Fresenius Kabi AB
SE-751 74 Uppsala, Sweden
Manufacturer
Fresenius Kabi AB
SE-751 74 Uppsala, Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium | Kabiven |
Denmark | Kabiven |
Finland | Kabiven |
France | Kabiven |
Germany | Kabiven |
Greece | Kabiven |
Iceland | Kabiven |
Ireland | Kabiven |
Italy | Kabiven |
Luxembourg | Kabiven |
Netherlands | Kabiven |
Portugal | Kabiven |
Spain | Kabiven |
Sweden | Kabiven |
United Kingdom | Kabiven |
Date of Last Revision of this Leaflet: April 2019
Detailed and up-to-date information on this product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals.
Warnings and Precautions for Use
To avoid the risk associated with too rapid perfusion rate, it is recommended to perform continuous and well-controlled perfusion, if possible using a volumetric pump.
Given the high risk of infection associated with the use of a central vein, strict aseptic precautions should be taken to avoid any contamination during catheter insertion and handling.
Serum levels of glucose, electrolytes, and osmolarity, as well as fluid balance, acid-base balance, and liver enzyme tests, should be monitored.
Upon any sign or symptom of anaphylactic reaction (such as fever, chills, skin rash, or dyspnea), perfusion should be immediately discontinued.
Kabiven should not be administered simultaneously with blood in the same infusion equipment due to the risk of pseudoagglutination.
Method of Administration
Intravenous route, infusion in a central vein.
Infusion Rate
The maximum infusion rate for glucose is 0.25 g/kg body weight/h.
The dosage of amino acids should not exceed 0.1 g/kg body weight/h.
The dosage of lipids should not exceed 0.15 g/kg body weight/h.
The infusion rate should not exceed 2.6 ml/kg body weight/hour (corresponding to 0.25 g glucose, 0.09 g amino acids, and 0.1 g lipids/kg body weight). The recommended infusion period is 12-24 hours.
Precautions for Disposal
Do not use the container if it is not intact. Use only if the glucose and amino acid solutions are clear and colorless or slightly yellow, and the lipid emulsion is white and homogeneous. The contents of the three separate chambers must be mixed before use and prior to any addition made through the additive port.
After separation of the peel seals, the bag should be inverted several times to ensure a homogeneous mixture that does not show evidence of phase separation.
For single use. Any remaining mixture after infusion should be discarded.
Compatibility
There are compatibility data with defined quantities of Dipeptiven, Supliven, Glycophos, Vitalipid Adult/Infant, and Soluvit products and generics of electrolytes in defined concentrations. When adding electrolytes, the quantities already present in the bag should be taken into account to meet the patient's clinical needs. The generated data support additions to the activated bag according to the summary table shown below:
Stable compatibility range for 8 days, i.e., 6 days of storage at 2-8°C followed by 48 hours at 20-25°C
Units | Maximum Total Content | ||||
Kabiven Bag Size | ml | 1026 | 1540 | 2053 | 2566 |
Additive | Volume | ||||
Dipeptiven | ml | 0 - 200 | 0 - 300 | 0 - 300 | 0 - 300 |
Supliven | ml | 0 - 10 | 0 - 10 | 0 - 20 | 0 - 20 |
Soluvit | vial | 0 - 1 | 0 - 1 | 0 - 2 | 0 - 2 |
Vitalipid Adult/Infant | ml | 0 - 10 | 0 - 10 | 0 - 20 | 0 - 20 |
Electrolyte Limit1 | Quantity per Bag | ||||
Sodium | mmol | ≤ 154 | ≤ 231 | ≤ 308 | ≤ 385 |
Potassium | mmol | ≤ 154 | ≤ 231 | ≤ 308 | ≤ 385 |
Calcium | mmol | ≤ 5 | ≤ 7.5 | ≤ 10 | ≤ 12.5 |
Magnesium | mmol | ≤ 5 | ≤ 7.5 | ≤ 10 | ≤ 12.5 |
Organic phosphate (Glycophos) | mmol | ≤ 15 | ≤ 22.5 | ≤ 30 | ≤ 37.5 |
- includes the quantities of all products
Note: This table is intended to indicate compatibility. It is not a dosage guide. For products with a trade name, before prescribing, consult the approved summary of product characteristics.
There are data on compatibility with other additives and the storage time of the different mixtures, available upon request.
Additions should be made aseptically.
Disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations.
Expiry Date after Mixing the Bag Chambers
After opening the peel seals, a chemical and physical stability of the mixed three-chamber bag has been demonstrated for 48 hours at 20-25°C, including the duration of administration. From a microbiological point of view, the product should be used immediately. If not used immediately, the storage time until use and the conditions of use are the responsibility of the user and normally should not exceed 24 hours at 2-8°C, unless the mixture has been made in controlled and validated aseptic conditions.
Expiry Date after Mixing with Additives
After breaking the peel seals and mixing the three solutions, additions can be made through the additive port. The physicochemical stability of the mixed three-chamber bag with additives has been demonstrated for a maximum of 8 days, i.e., 6 days at 2-8°C followed by 48 hours at 20-25°C, including the duration of administration.
From a microbiological point of view, the product should be used immediately after making the additions. If not used immediately, the storage time until use and the conditions of use are the responsibility of the user and normally should not exceed 24 hours at 2-8°C, unless the mixture has been made in controlled and validated aseptic conditions.
Kabiven Instructions for Use
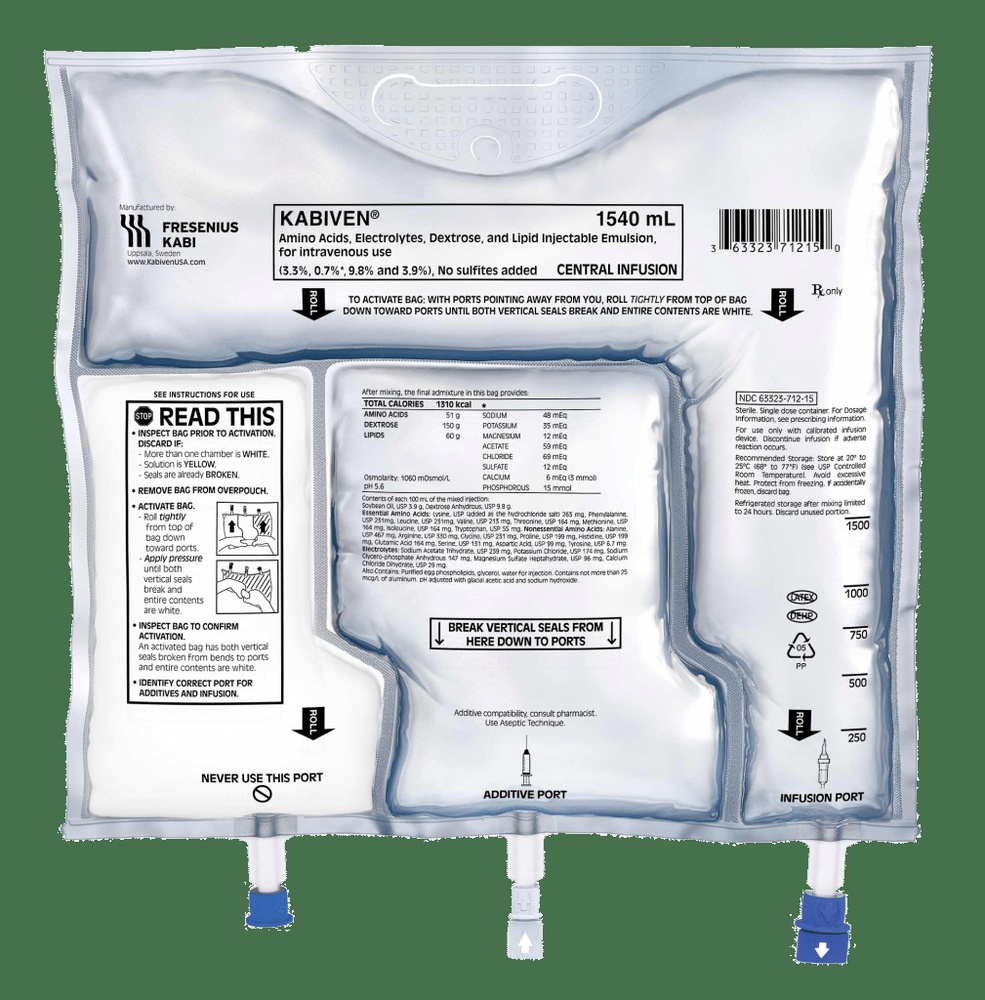
The Bag
- Notches on the overbag
- Hanger
- Ring for hanging the bag
- Peel seals
- Port without outlet (only used during manufacturing)
- Additive port
- Infusion port
- Oxygen absorber
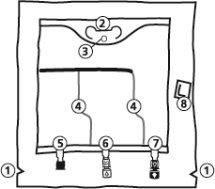
- Removal of the overbag
- To remove the overbag, hold it in a horizontal position and tear along the notch towards the ports along the top edge (A).
- Then, simply tear along the container; separate the overbag and discard it along with the oxygen absorber (B).
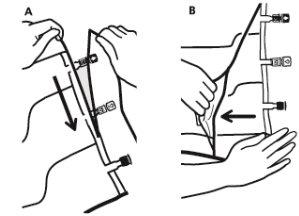
- Mixing
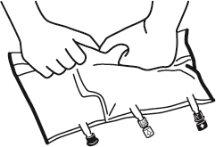
- Place the bag on a flat surface.
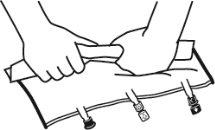
- Roll the bag from the hanger part towards the port part, first with the right hand and then applying constant pressure with the left hand until the vertical peel seals have opened. The vertical peel seals open due to the liquid pressure. The peel seals can also be opened before removing the overbag.
Note:The liquids mix easily even if the horizontal seal remains closed.
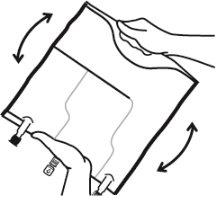
- Mix the contents of the three chambers by inverting the bag three times until the components are completely mixed.
- Final Preparation
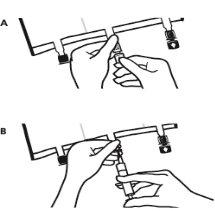
- Place the bag back on a flat surface. Just before injecting the additives, break the white additive port by the arrow mark (A).
Note:The additive port membrane is sterile
- Hold the base of the additive port. Insert the needle, inject the additives (of known compatibility) through the center of the injection point (B).
- Mix completely between each addition, inverting the bag three times. Use syringes with 18-23 gauge needles and a maximum length of 40 mm.
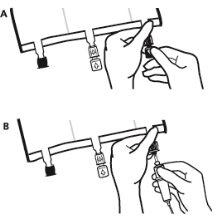
- Just before inserting the infusion set, break the blue infusion port by the arrow mark (A).
Note:The infusion port membrane is sterile
- Use a non-vented infusion equipment or close the air inlet of the vented equipment.
- Hold the base of the infusion port.
- Insert the spike through the infusion port. The spike should be fully inserted to ensure its retention.
Note:The inside of the infusion port is sterile
- Hanging the Bag
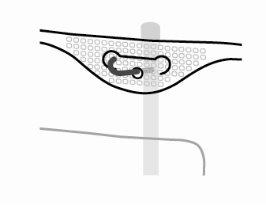
Hang the bag by the ring under the hanger.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KABIVEN EMULSION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for KABIVEN EMULSION FOR INFUSION
Discuss questions about KABIVEN EMULSION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















