INTESTIFALK 4 mg SUPPOSITORIES

How to use INTESTIFALK 4 mg SUPPOSITORIES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Intestifalk 4mg suppositories
budesonide Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Intestifalk and what is it used for
- What you need to know before you start using Intestifalk
- How to use Intestifalk
- Possible side effects
- Storage of Intestifalk
- Contents of the pack and other information
1. What is Intestifalk and what is it used for
Intestifalk contains the active substance budesonide, a locally acting steroid used to treat inflammatory diseases of the intestine.
Intestifalk is indicated in adult patients for the treatment of acute episodes of inflammatory bowel disease (ulcerative proctitis).
2. What you need to know before you start using Intestifalk
Do not use Intestifalk
- if you are allergic to budesonide or any of the other ingredients of this medicine (listed in section 6).
- if you have severe liver disease (liver cirrhosis).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Intestifalk if you have:
- tuberculosis
- high blood pressure
- diabetes or if a family member has been diagnosed with diabetes
- bone fragility (osteoporosis)
- stomach or duodenal ulcers (peptic ulcer)
- increased pressure in the eye (glaucoma) or eye problems such as cataracts, or if a family member has been diagnosed with glaucoma
- liver problems
- kidney problems
Additional precautions during treatment with Intestifalk
- Tell your doctor if you have an infection. The symptoms of some infections may be atypical or less pronounced.
- Stay away from people who have chickenpox or herpes zoster (shingles) if you have not had them before. They could affect you severely. If you come into contact with chickenpox or herpes zoster, see your doctor immediately.
- Tell your doctor if you have not had measles.
- If you need to receive any vaccine during treatment with this medicine, tell your doctor beforehand.
- Tell your doctor that you are using Intestifalk in case of scheduled surgery.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Intestifalk may affect the results of tests performed by your doctor or in the hospital. Tell your doctor that you are using Intestifalk before undergoing any tests.
The use of the medicine Intestifalk 4 mg suppositories may result in positive doping tests.
Elderly people
Close monitoring for the occurrence of side effects is required in elderly patients.
Children and adolescents
Intestifalk must not be used in children and adolescents under 18 years of age. The use of this medicine has not been studied in patients under 18 years of age.
Other medicines and Intestifalk
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. In particular:
- cardiac glycosidessuch as digoxin (medicines used to treat heart disorders)
- diuretics(to eliminate excess fluid from your body)
- ketoconazole or itraconazole(to treat fungal infections)
- clarithromycin, an antibioticused to treat infections
- carbamazepine(used in the treatment of epilepsy)
- rifampicin(to treat tuberculosis)
- estrogens or oral contraceptives
Some medicines may increase the effects of Intestifalk, so your doctor may monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Using Intestifalk with food and drinks
Do not drink grapefruit juice during treatment with this medicine, as it may alter its effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
You should only use Intestifalk during pregnancy if your doctor has told you to.
Budesonide passes into breast milk in small amounts. If you are breastfeeding, you should only use Intestifalk if your doctor has told you to.
Driving and using machines
Intestifalk is unlikely to affect your ability to drive or use machines.
3. How to use Intestifalk
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
Dosage
The recommended dose is one Intestifalk 4 mg suppository per day.
Method of administration
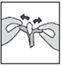
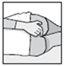
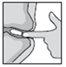
This medicine can only be used rectally, so it must be inserted into the anus. DO NOT take it orally.
Intestifalk should be administered at bedtime, so that the suppository remains in place for as long as possible.
How to insert the suppository
| |
| |
|
|
|
|
|
|
|
Duration of treatment
Your doctor will decide how long you need to use this medicine. The duration of treatment depends on the nature of your disease. Acute episodes of inflammatory bowel disease (ulcerative proctitis) usually subside after 6-8 weeks.
If you use more Intestifalk than you should
If you have used too many suppositories on one occasion, use the next dose as prescribed. Do not use a smaller dose. If you are unsure, contact your doctor to decide what to do; if possible, take the package and package leaflet with you.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use IntestifalkIf you forget a dose, continue treatment with the prescribed dose. Do not use a double dose to make up for the forgotten dose.
If you stop using Intestifalk
Talk to your doctor if you want to stop or conclude your treatment prematurely. Continue using your medicine until your doctor tells you to, even if you start to feel better.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported during the use of Intestifalk:
Very common: may affect up to 1 in 10 people
- decrease in cortisol hormone levels in the blood
Uncommon: may affect up to 1 in 100 people
- reduction of adrenal gland function (small glands located next to the kidneys)
- headache
- flushing
- abdominal pain, flatulence, severe abdominal pain due to acute pancreatitis
- skin rash
- changes in menstrual cycle such as irregular periods
The following side effects have been reported, which are typical of medicines similar to Intestifalk (corticosteroids) and may therefore also occur with this medicine. The frequency of these side effects is currently unknown:
- increased risk of infections
- Cushing's syndrome, which is associated with an excess of corticosteroids and causes "moon face", weight gain, high blood sugar levels (hyperglycemia), fluid accumulation in tissues (e.g., swollen legs), low potassium levels in the blood (hypokalemia), unwanted body hair in women, impotence, stretch marks on the skin, acne
- mood changes, such as depression, irritability, or euphoria
- restlessness with increased physical activity, anxiety, aggression
- blurred vision
- increased risk of blood clots, inflammation of blood vessels, high blood pressure
- indigestion, stomach and duodenal ulcers, constipation
- allergic skin rash, red spots due to bleeding in the skin, delayed wound healing, skin reactions such as contact dermatitis, bruising
- muscle and joint pain, muscle weakness, muscle twitching (fasciculations)
- bone weakness (osteoporosis), bone damage due to poor blood circulation (osteonecrosis)
- fatigue and general feeling of being unwell
These side effects are typical of steroid medicines. They may occur depending on the dose, duration of treatment, whether you have previously taken or are taking other cortisone preparations, and your individual sensitivity.
If you have received treatment with a more potent cortisone preparation before starting treatment with Intestifalk 4 mg suppositories, your symptoms may recur when you switch medicines. If this happens, contact your doctor.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly to the Spanish Medicines Vigilance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Intestifalk 4 mg suppositories
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date is the last day of the month stated.
Do not store above 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Intestifalk
- The active substanceis budesonide.
- The other ingredientsare ascorbyl palmitate E 304(i) and semi-synthetic solid glycerides.
Appearance and pack contents
Intestifalk are white, torpedo-shaped suppositories (approximately 2 cm long) with a smooth surface.
Intestifalk is available in packs containing 12, 30, 55, or 60 suppositories.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Dr. Falk Pharma GmbH
Leinenweberstr. 5
79108 Freiburg
Germany
Tel.: +49 (0)761 1514-0
Fax: +49 (0)761 1514-321
E-mail: [email protected]
You can obtain further information on this medicine from the representative of the marketing authorisation holder:
Dr. Falk Pharma España
Camino de la Zarzuela, 19
28023 Madrid
Spain
This medicine is authorised in the Member States of the European Economic Area and in the following countries under the names:
Germany, Austria, Belgium, Denmark, Slovakia, Slovenia, Finland, Hungary, Ireland, Latvia, Lithuania, Luxembourg, Norway, Netherlands, Portugal, Czech Republic, Romania, Sweden: Budenofalk.
Cyprus, Croatia: Budosan.
Greece: Budenofalk procto.
Italy: Intesticortproct.
France: Mikicort.
Spain: Intestifalk.
Date of last revision of this package leaflet: December 2023
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INTESTIFALK 4 mg SUPPOSITORIESDosage form: RECTAL LIQUID, 2 mg budesonideActive substance: budesonideManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE CAPSULE, 3 mg budesonideActive substance: budesonideManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: RECTAL SEMISOLID, 2 mgActive substance: budesonideManufacturer: Dr. Falk Pharma GmbhPrescription required
Online doctors for INTESTIFALK 4 mg SUPPOSITORIES
Discuss questions about INTESTIFALK 4 mg SUPPOSITORIES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions