INTESTIFALK 2 mg/DOSE RECTAL FOAM

How to use INTESTIFALK 2 mg/DOSE RECTAL FOAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Prospectus: information for the user
Intestifalk 2 mg/dose rectal foam
budesonide
Read the entire prospectus carefully before starting to use this medication, as it contains important information for you.
- Keep this prospectus, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this prospectus. See section 4.
Contents of the prospectus
- What Intestifalk rectal foam is and what it is used for
- What you need to know before starting to use Intestifalk rectal foam
- How to use Intestifalk rectal foam
- Possible side effects
- Storage of Intestifalk rectal foam
Contents of the package and additional information
1. What Intestifalk rectal foam is and what it is used for
Intestifalk rectal foam contains the active ingredient budesonide, a local steroid anti-inflammatory for treating inflammatory diseases of the intestine.
Intestifalk rectal foam is indicated for the treatment of:
a inflammatory disease of the rectum and large intestine (sigmoid colon) called ulcerative colitis by doctors.
2. What you need to know before starting to use Intestifalk rectal foam
Do not use Intestifalk rectal foam
- If you are allergic to budesonide or any of the other components of this medication (listed in section 6).
- If you have severe liver disease (liver cirrhosis).
Warnings and precautions
Consult your doctor before starting to use Intestifalk rectal foam if you have:
- tuberculosis.
- high blood pressure.
- diabetes or if a family member has been diagnosed with diabetes.
- bone fragility (osteoporosis).
- stomach or small intestine ulcers (peptic ulcer).
- increased eye pressure (glaucoma) or eye problems such as cataracts or if a family member has been diagnosed with glaucoma.
- severe liver problems.
The typical effects of cortisone preparations may appear, affecting all parts of the body, particularly if you use Intestifalk rectal foam at high doses and for prolonged periods (see section 4. Possible side effects).
Additional precautions during treatment with Intestifalk rectal foam:
- Tell your doctor if you have an infection. The symptoms of some infections may be atypical or less marked.
- Stay away from people who have chickenpox or shingles (herpes zoster) if you have not had them before. They can affect you severely. If you come into contact with chickenpox or shingles, see your doctor immediately.
- Tell your doctor if you have not had measles.
- If you need to receive a vaccine during treatment with this medication, inform your doctor beforehand.
- Tell your doctor that you are using Intestifalk rectal foam in case of scheduled surgery.
- If you have been treated with a more potent cortisone preparation before starting treatment with Intestifalk rectal foam, your symptoms may reappear when changing medications. If this happens, inform your doctor.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Using Intestifalk rectal foam with other medications
Tell your doctor or pharmacist if you are using or have recently used other medications, or if you may need to use them. In particular:
- cardiac glycosidessuch as digoxin (medications used to treat heart conditions)
- diuretics(medications used to treat excess fluid in your body)
- ketoconazole or itraconazole(for treating fungal infections)
- antibiotics, medications for treating infections (such as clarithromycin)
- carbamazepine(used in the treatment of epilepsy)
- rifampicin(for treating tuberculosis)
- estrogens or oral contraceptives
Intestifalk rectal foam may alter the results of tests performed by your doctor or in a hospital. Inform your doctor that you are using Intestifalk rectal foam before undergoing any tests.
Some medications may increase the effects of Intestifalk rectal foam, so your doctor will monitor you closely if you are taking these medications (including some for HIV: ritonavir, cobicistat).
Using Intestifalk rectal foam with food and beverages
Do notdrink grapefruit juiceduring treatment with Intestifalk rectal foam, as it may alter its effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication.
You should only use Intestifalk rectal foam during pregnancy if your doctor advises you to do so.
Budesonide passes into breast milk in small amounts. If you are breastfeeding, you should only use Intestifalk rectal foam if your doctor advises you to do so.
Driving and using machines
It is unlikely that Intestifalk rectal foam will affect your ability to drive or use machines.
Intestifalk rectal foam contains propylene glycol, cetyl alcohol, and cetearyl alcohol
This medication contains 600.3 mg of propylene glycol in each Intestifalk rectal foam application. Propylene glycol may cause skin irritation.
Cetyl alcohol and cetearyl alcohol (component of the emulsifying wax) may cause local skin reactions (such as contact dermatitis).
.
3. How to use Intestifalk rectal foam
Follow your doctor's instructions for administering this medication exactly. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is:
Dosage for adults over 18 years
The normal dose is 1 spray application, once a day in the morning or at bedtime. The best results are achieved using Intestifalk rectal foam after emptying your intestine.
Use in children and adolescents
Intestifalk rectal foam should not be used in children under 18 years, as there is very limited experience with this age group.
Method of administration
This medication can only be used rectally, so it must be inserted through the anus. It is not intended for oral use. Do not ingest.
Aerosol package illustration
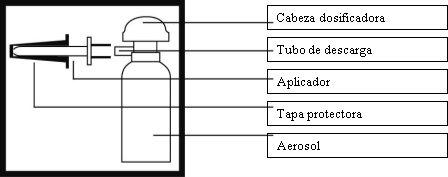
The applicator and its protective cap come in a special mold. Please hold the mold firmly and pull the applicator with force.
Preparation for use of the foam:
 Connect the applicator firmly to the discharge tube of the package. Shake the package for approximately 15 seconds to mix the contents.
Connect the applicator firmly to the discharge tube of the package. Shake the package for approximately 15 seconds to mix the contents.

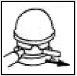 Before using for the first time, remove the safety lock (plastic flap) from the dosing head.
Before using for the first time, remove the safety lock (plastic flap) from the dosing head.
 Turn the package head until the semicircular notch located below the head is in line with the applicator. Now the aerosol is ready to use.
Turn the package head until the semicircular notch located below the head is in line with the applicator. Now the aerosol is ready to use.
Using the foam:
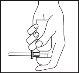 Place your index finger on the top of the dosing head and turn the aerosol upside down. Note that the aerosol only works properly when held with the dosing head facing down as vertically as possible.
Place your index finger on the top of the dosing head and turn the aerosol upside down. Note that the aerosol only works properly when held with the dosing head facing down as vertically as possible.
| Place one foot on a chair or stool and lie on your side with your lower leg straight and your upper leg bent to maintain balance. Insert the applicator into your rectum as far as possible. Press the dosing head completely once and then release it very slowly; the foam comes out of the aerosol when you release the dosing head. Keep the applicator in place for 10 to 15 seconds before removing it. This ensures that the full dose is administered and that no foam is spilled. |
 After administering the foam, separate the applicator and discard it with your household trash using one of the plastic bags provided. For the next application, use a new applicator.
After administering the foam, separate the applicator and discard it with your household trash using one of the plastic bags provided. For the next application, use a new applicator.
To avoid unintentional loss of foam between applications, turn the dosing head so that the semicircular notch is facing away from the discharge tube.
- Wash your hands and try not to empty your intestine until the next morning.
- If you are going to the hospital or visiting another doctor or dentist, tell them that you are using this medication.
Duration of treatment
The duration of treatment depends on the nature of your disease.
Your doctor will decide how long you need to continue using the medication.
Mild acute episodes of inflammatory bowel disease (ulcerative colitis) usually subside after 6-8 weeks.
If you feel that the effect of Intestifalk rectal foam is too strong or too weak, consult your doctor.
If you use more Intestifalk rectal foam than you should
If you have used too much medication on one occasion, use the next dose as prescribed. Do not use a smaller amount. If you have any doubts, contact your doctor to decide what to do, and if possible, take the box and prospectus with you.

If you forget to use Intestifalk rectal foam
If you forget a dose, continue treatment with the prescribed dose. Do not use a double dose to make up for the forgotten dose.
If you stop treatment with Intestifalk rectal foam
Talk to your doctor if you want to stop or conclude your treatment early. It is essential that you do not stop using your medication suddenly, as this could make you ill. Continue using your medication until your doctor tells you to do so, even if you start to feel better.
If you have any other questions about using this medication, ask your doctor or pharmacist.
.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
If you experience any of the following symptoms after using this medication, you should contact your doctor immediately:
- Infection
- Headache
- Changes in behavior such as depression, irritability, euphoria, restlessness, anxiety, or aggression.
The following side effects have also been reported:
Common: may affect up to 1 in 10 people
- Burning or pain in the rectum
- Cushing's syndrome - for example, moon face, weight gain, reduced glucose tolerance, high blood sugar, high blood pressure, fluid retention in tissues (e.g., swollen legs), increased potassium excretion (hypokalemia), irregular periods in women, unwanted hirsutism in women, impotence, abnormal laboratory findings (reduced adrenal function), formation of red stretch marks on the skin (striae), acne
- Indigestion, irritable stomach (dyspepsia)
- Increased risk of infection
- Muscle and joint pain, muscle weakness, muscle cramps
- Bone fragility (osteoporosis)
- Headache
- Mood changes, such as depression, irritation, or euphoria
- Rash due to hypersensitivity reactions, red spots due to bleeding in the skin, delayed wound healing, local skin reactions such as contact dermatitis
Uncommon: may affect up to 1 in 100 people
- Increased appetite
- Changes in blood tests (increased erythrocyte sedimentation rate, increased white blood cell count)
- Nausea, abdominal pain, gas, bloating or tingling in the abdomen, anal fissure, mouth ulcers, urgent need to empty the intestine, rectal bleeding.
- Stomach or small intestine ulcers
- Changes in liver function parameters
- Changes in pancreatic function, variations in adrenal hormones
- Urinary tract infections
- Dizziness, changes in smell
- Insomnia, restlessness with increased physical activity, anxiety
- Increased sweating, weakness
Rare: may affect up to 1 in 1,000 people
- Blurred vision.
- Pancreatitis
- Bone loss due to poor blood circulation (osteonecrosis)
- Aggression
- Bruising
Very rare: may affect up to 1 in 10,000 people
- Growth retardation in children
- Constipation
- Increased intracranial pressure, possibly with increased eye pressure
(inflammation of the optic disc) in adolescents
- Increased risk of thrombosis, inflammation of blood vessels (associated with
withdrawal of cortisone after long-term treatment)
- Fatigue, feeling of general malaise
These side effects are typical of steroid medications and most of them are also predictable for treatments with other steroids. They may appear depending on the dose, duration of treatment, whether you have followed or are following treatment with other cortisone preparations, and your personal sensitivity.
Some of the adverse reactions were only reported after long-term oral administration of budesonide.
Due to its local action, the risk of adverse reactions to Intestifalk 2 mg rectal foam is generally lower than treatment with systemic glucocorticosteroids (whose action extends throughout the body).
If you have been treated with a more potent cortisone preparation before starting treatment with Intestifalk rectal foam, your symptoms may reappear when changing medications.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Intestifalk rectal foam
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the box and pressurized package. The expiration date is the last day of the month indicated.
The package contents should be used within 4 weeks of opening.
Do not store above 25°C.
Do not refrigerate or freeze.
The package is pressurized and contains a flammable propellant.
Do not expose to temperatures above 50°C, protect from direct sunlight.
Do not puncture or burn, even when empty.
Medications should not be disposed of through wastewater or household waste. Deposit the packages and medications you no longer need at the SIGRE collection point in your pharmacy. Ask your pharmacist how to dispose of packages and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Intestifalk Rectal Foam
The active ingredient is budesonide. Each dose contains 2 mg of budesonide.
The other components are cetyl alcohol, emulsifying wax, purified water, disodium edetate, macrogol stearate ether, propylene glycol, citric acid monohydrate, and n-butane, isobutane, and propane as propellants.
Appearance of the Product and Package Contents
Intestifalk rectal foam is a firm, creamy foam with a white to grayish-white color, presented in a pressurized container.
Intestifalk rectal foam is available in boxes with 1 pressurized container, 14 applicators, and 14 plastic bags or in boxes with 2 pressurized containers, 28 applicators, and 28 plastic bags for hygienic disposal of the applicators.
Not all containers may be marketed.
Marketing Authorization Holder and Manufacturer
Dr. Falk Pharma GmbH
Leinenweberstr. 5
79108 Freiburg
Germany
TEL +49 (0) 761 / 1514-0
E-mail: [email protected]
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
Spain
Dr. Falk Pharma España
Camino de la Zarzuela, 19
28023 Madrid
This medicinal product is authorized in the Member Statesof the European Economic Areaunder the following names:
Denmark, Finland, Greece, Ireland, Romania, Sweden, and United Kingdom:
Budenofalk.
Austria: Budo-San.
Italy: Intesticort.
Spain: Intestifalk.
Date of the Last Revision of this Leaflet:September 2020
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price80.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INTESTIFALK 2 mg/DOSE RECTAL FOAMDosage form: RECTAL LIQUID, 2 mg budesonideActive substance: budesonideManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE CAPSULE, 3 mg budesonideActive substance: budesonideManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: CAPSULE, 3 mg budesonideActive substance: budesonideManufacturer: Dr. Falk Pharma GmbhPrescription required
Online doctors for INTESTIFALK 2 mg/DOSE RECTAL FOAM
Discuss questions about INTESTIFALK 2 mg/DOSE RECTAL FOAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions